Emergency contraception (morning after pill, IUD)

Emergency contraception can prevent pregnancy after unprotected sex or if your contraceptive method has failed – for example, a condom has split or you've missed a pill. There are two types:
- the emergency contraceptive pill (sometimes called the morning after pill)
- the IUD (intrauterine device, or coil)
- At a glance: emergency contraception
- The emergency pill
- The IUD as emergency contraception
- Where to get emergency contraception
- Contraception for the future
There are two kinds of emergency contraceptive pill. Levonelle has to be taken within 72 hours (three days) of sex, and ellaOne has to be taken within 120 hours (five days) of sex. Both pills work by preventing or delaying ovulation (release of an egg).
The IUD can be inserted into your uterus up to five days after unprotected sex, or up to five days after the earliest time you could have ovulated. It may stop an egg from being fertilised or implanting in your womb.
Emergency contraception does not protect against sexually transmitted infections (STIs).
At a glance: facts about emergency contraception
- Both types of emergency contraception are effective at preventing pregnancy if they are used soon after unprotected sex. Less than 1% of women who use the IUD get pregnant, whereas pregnancies after the emergency contraceptive pill are not as rare. It’s thought that ellaOne is more effective than Levonelle.
- The sooner you take Levonelle or ellaOne, the more effective it will be.
- Levonelle or ellaOne can make you feel sick, dizzy or tired, or give you a headache, tender breasts or abdominal pain.
- Levonelle or ellaOne can make your period earlier or later than usual.
- If you’re sick (vomit) within two hours of taking Levonelle, or three hours of taking ellaOne, seek medical advice as you will need to take another dose or have an IUD fitted.
- If you use the IUD as emergency contraception, it can be left in as your regular contraceptive method.
- If you use the IUD as a regular method of contraception, it can make your periods longer, heavier or more painful.
- You may feel some discomfort when the IUD is put in – painkillers can help to relieve this.
- There are no serious side effects of using emergency contraception.
- Emergency contraception does not cause an abortion.
The emergency pill
How the emergency pill works
How effective the emergency pill is at preventing pregnancy
How it affects your period
Who can use the emergency pill
During pregnancy and breastfeeding
If you're already using the pill, patch, vaginal ring or injection
Side effects of the emergency pill
The emergency pill and other medicines
Can I get the emergency pill in advance?
How the emergency pill works
Levonelle
Levonelle contains levonorgestrel, a synthetic version of the natural hormone progesterone. In a woman’s body, progesterone plays a role in ovulation and preparing the uterus for accepting a fertilised egg.
It’s not known exactly how Levonelle works, but it’s thought to work primarily by preventing or delaying ovulation. You can take Levonelle more than once in a menstrual cycle. It does not interfere with your regular method of contraception.
ellaOne
ellaOne contains ulipristal acetate, which means that it stops progesterone working normally. It prevents pregnancy mainly by preventing or delaying ovulation. ellaOne may prevent other types of hormonal contraception from working for a week after use, and it’s not recommended for use more than once in a menstrual cycle.
ellaOne used to be available only on prescription, but it is now available to buy in some pharmacies.
Levonelle and ellaOne do not protect you against pregnancy during the rest of your menstrual cycle and are not intended to be a regular form of contraception. Using the emergency contraceptive pill repeatedly can disrupt your natural menstrual cycle.
How effective is the emergency pill at preventing pregnancy?
- most GP surgeries
- community contraception clinics
- some GUM clinics
- sexual health clinics
- some young people's services
Find a clinic near you
It can be difficult to know how many pregnancies the emergency pill prevents, because there is no way to know for sure how many women would have got pregnant if they did not take it.
A trial undertaken by the World Health Organization (WHO) indicated that levonorgestrel (the drug in Levonelle) prevented:
- 95% of expected pregnancies when taken within 24 hours of sex
- 85% if taken within 25-48 hours
- 58% if taken within 49-72 hours
More recent studies suggest that the prevention rate might be lower, but still substantial.
A study published in 2010 showed that of 1,696 women who received the emergency pill within 72 hours of sex, 37 became pregnant (1,659 did not). Of 203 women who took the emergency pill between 72 and 120 hours after unprotected sex, there were three pregnancies.
How it affects your period
After taking the emergency contraceptive pill, most women will have a normal period at the expected time. However, you may have your period later or earlier than normal.
If your period is more than seven days late, or is unusually light or short, contact your GP as soon as possible to check for pregnancy.
Who can use the emergency pill?
Most women can use the emergency contraceptive pill. This includes women who cannot usually use hormonal contraception, such as the combined pill and contraceptive patch.
Levonelle
The WHO does not identify any medical condition that would mean a woman shouldn’t use Levonelle.
ellaOne
The Faculty of Sexual and Reproductive Healthcare (FSRH) advises that ellaOne should not be used by women who:
- may already be pregnant
- are allergic to any of the components of the drug
- have severe asthma that is not properly controlled by steroids
- have hereditary problems with lactose metabolism
ellaOne will not be effective in women who are taking liver enzyme-inducing medication. For more information, read The emergency pill and other medicines.
Pregnancy and breastfeeding
Levonelle
There is no evidence that Levonelle harms a developing baby. It can be used even if there has been an earlier episode of unprotected sex in the menstrual cycle in addition to the current episode. Levonelle can be taken while breastfeeding. Although small amounts of the hormones contained in the pill may pass into your breast milk, it is not thought to be harmful to your baby.
ellaOne
There is limited information on the safety of ellaOne in pregnancy. The FSRH does not support the use of ellaOne if a woman might already be pregnant. The safety of ellaOne during breastfeeding is not yet known. The manufacturer recommends that you do not breastfeed for one week after taking this pill.
If you are already using the pill, patch, vaginal ring or contraceptive injection
If you need to take the emergency pill because you:
- forgot to take some of your regular contraceptive pills, or
- did not use your contraceptive patch or vaginal ring correctly, or
- were late having your contraceptive injection
then you should:
- take your next contraceptive pill, apply a new patch or insert a new ring within 12 hours of taking the emergency pill
You should then continue taking your regular contraceptive pill as normal.
If you have taken Levonelle, you will need to use additional contraception, such as condoms, for:
- the next seven days if you use the patch, ring, combined pill or injection
- the next two days if you use the progestogen-only pill
If you have taken ellaOne, you will need to use additional contraception, such as condoms, for:
- the next 14 days if you use the patch, ring, combined pill or injection
- the next nine days if you use the progestogen-only pill
What are the side effects of using the emergency pill?
Taking the emergency contraceptive pill has not been shown to cause any serious or long-term health problems. However, it can sometimes have side effects. Common side effects include:
- abdominal (tummy) pain
- headache
- irregular menstrual bleeding (spotting or heavy bleeding) before your next period is due
- feeling sick
- tiredness
Less common side effects include:
- breast tenderness
- dizziness
- headache
- vomiting (seek medical advice if you vomit within two hours of taking Levonelle, or three hours of taking ellaOne, as you will need to take another dose or have an IUD fitted)
If you are concerned about any symptoms after taking the emergency contraceptive pill, contact your GP or speak to a nurse at a sexual health clinic. You should talk to a doctor or nurse if:
- you think you might be pregnant
- your next period is more than seven days late
- your period is shorter or lighter than usual
- you have any sudden or unusual pain in your lower abdomen (this could be a sign of an ectopic pregnancy, where a fertilised egg implants outside the womb – this is rare but serious, and needs immediate medical attention)
The emergency pill and other medicines
The emergency contraceptive pill may interact with other medicines. These include:
- the herbal medicine St John’s Wort
- some medicines used to treat epilepsy
- some medicines used to treat HIV
- some medicines used to treat tuberculosis (TB)
- medication such as omeprazole (an antacid) to make your stomach less acidic
ellaOne cannot be used if you are already taking one of these medicines, as it may not be effective.
Levonelle may still be used, but the dose may need to be increased – your doctor or pharmacist can advise on this.
There should be no interaction between the emergency pill and most antibiotics. Two enzyme-inducing antibiotics (called rifampicin and rifabutin), used to treat or prevent meningitis or TB, may affect ellaOne while they’re being taken and for 28 days afterwards.
If you want to check that your medicines are safe to take with the emergency contraceptive pill, ask your GP or a pharmacist. You should also read the patient information leaflet that comes with your medicines.
Can I get the emergency contraceptive pill in advance?
You may be able to get the emergency contraceptive pill in advance of having unprotected sex if:
- you are worried about your contraceptive method failing
- you are going on holiday
- you cannot get hold of emergency contraception easily
Ask your GP or nurse for further information on getting advance emergency contraception.
The IUD as emergency contraception
An intrauterine device (IUD)
- How the IUD works
- How effective the IUD is at preventing pregnancy
- Who can use the IUD
- During pregnancy and breastfeeding
- Side effects of using the IUD
- The IUD and other medicines
How the IUD works
The intrauterine device (IUD) is a small, T-shaped contraceptive device made from plastic and copper. It’s inserted into the uterus by a trained health professional. It may prevent an egg from implanting in your womb or being fertilised.
If you’ve had unprotected sex, the IUD can be inserted up to five days afterwards, to prevent pregnancy. It’s more effective at preventing pregnancy than the emergency pill, and it does not interact with any other medication.
You can also choose to have the IUD left in as an ongoing method of contraception.
How effective the IUD is at preventing pregnancy
There are several types of IUD. Newer ones have more copper and are more than 99% effective. Fewer than two women in 100 who use a newer IUD over five years will get pregnant. IUDs with less copper in them are less effective than this, but are still effective. The IUD is more effective than the emergency pill at preventing pregnancy after unprotected sex.
Who can use the IUD
Most women can use an IUD, including women who have never been pregnant and those who are HIV positive. Your GP or clinician will ask about your medical history to check if an IUD is suitable for you.
You should not use an IUD if you have:
- an untreated STI or a pelvic infection
- certain abnormalities of the womb or cervix
- any unexplained bleeding from your vagina – for example, between periods or after sex
Women who have had an ectopic pregnancy or recent abortion, or who have an artificial heart valve, must consult their GP or clinician before having an IUD fitted.
Pregnancy and breastfeeding
The IUD should not be inserted if there is a risk that you may already be pregnant – for example, if you have had previous unprotected sex in the same menstrual cycle. The IUD can be used safely if you’re breastfeeding.
What are the side effects of the IUD
Complications after having an IUD fitted are rare, but can include pain, infection, damage to the womb or expulsion (the IUD coming out of your womb). If you use the IUD as an ongoing method of regular contraception, it may make your periods longer, heavier or more painful.
The IUD and other medicines
The emergency IUD will not react with any other medication.
Where can I get emergency contraception?
You can get the emergency contraceptive pill and the IUD for free from:
- a GP surgery that provides contraception (some GP surgeries may not provide the IUD)
- a contraception clinic
- a sexual health clinic (find sexual health services near you)
- some genitourinary medicine (GUM) clinics
- some young people's clinics (call 0800 567123)
You can also get the emergency contraceptive pill free from:
- some pharmacies (find pharmacies near you)
- most NHS walk-in centres and minor injuries units
- some Accident & Emergency departments
The doctor or nurse you see may ask for the following information:
- when you have had unprotected sex in your current menstrual cycle
- the date of the first day of your last period and the usual length of your cycle
- details of any contraceptive failure (such as how many pills you may have missed, and when)
- if you've used any medications that may affect your contraception
You can buy the emergency contraceptive pill from most pharmacies if you're aged 16 or over (you need to be 18 or over to buy ellaOne) and from some organisations such as bpas or Marie Stopes. The cost varies, but it will be around £30.
Contraception for the future
If you're not using a regular method of contraception, you might consider doing so in order to lower the risk of unintended pregnancy. Long-acting reversible contraception (LARC) offers the most reliable protection against pregnancy, and you don't have to think about it every day or each time you have sex.
LARC methods are the:
- injection
- implant
- IUS
- IUD
The contraceptive injection
A woman can get pregnant if a man’s sperm reaches one of her eggs (ova). Contraception tries to stop this happening by keeping the egg and sperm apart or by stopping egg production. One method of contraception is the injection.
- At a glance: the contraceptive injection
- How it works
- Who can use it
- Advantages and disadvantages
- Risks
- Where you can get it
There are three types of contraceptive injections in the UK: Depo-Provera, which lasts for 12 weeks, Sayana Press, which lasts for 13 weeks, and Noristerat, which lasts for eight weeks. The most popular is Depo-Provera. Noristerat is usually used for only short periods of time – for example, if your partner is waiting for a vasectomy.
The injection contains progestogen. This thickens the mucus in the cervix, stopping sperm reaching an egg. It also thins the womb lining and, in some, prevents the release of an egg.
At a glance: the contraceptive injection
- If used correctly, the contraceptive injection is more than 99% effective. This means that less than one woman in 100 who use the injection will become pregnant in a year.
- The injection lasts for eight, 12 or 13 weeks (depending on the type), so you don't have to think about contraception every day or every time you have sex.
- It can be useful for women who might forget to take the contraceptive pill every day.
- It can be useful for women who can't use contraception that contains oestrogen.
- It's not affected by medication.
- The contraceptive injection may provide some protection against cancer of the womb and pelvic inflammatory disease.
- Side effects can include weight gain, headaches, mood swings, breast tenderness and irregular bleeding. The injection can't be removed from your body, so if you have side effects they'll last as long as the injection and for some time afterwards.
- Your periods may become more irregular or longer, or stop altogether (amenorrhoea). Treatment is available if your bleeding is heavy or longer than normal – talk to your doctor or nurse about this.
- It can take up to one year for your fertility to return to normal after the injection wears off, so it may not be suitable if you want to have a baby in the near future.
- Using Depo-Provera affects your natural oestrogen levels, which can cause thinning of the bones.
- The injection does not protect against sexually transmitted infections (STIs). By using condoms as well as the injection, you'll help to protect yourself against STIs.
How the injection works
- most GP surgeries
- community contraception clinics
- some GUM clinics
- sexual health clinics
- some young people's services
Find a clinic near you
The contraceptive injections Depo-Provera and Noristerat are usually given into a muscle in your bottom, although sometimes may be given in a muscle in your upper arm. Sayana Press is given under the skin (subcutaneously) rather than into a muscle, in the abdomen or thigh.
The contraceptive injection works in the same way as the implant. It steadily releases the hormone progestogen into your bloodstream. Progestogen is similar to the natural hormone progesterone, which is released by a woman's ovaries during her period.
The continuous release of progestogen:
- stops a woman releasing an egg every month (ovulation)
- thickens the mucus from the cervix (neck of the womb), making it difficult for sperm to pass through to the womb and reach an unfertilised egg
- makes the lining of the womb thinner, so that it is unable to support a fertilised egg
The injection can be given at any time during your menstrual cycle, as long as you and your doctor are reasonably sure you are not pregnant.
When it starts to work
If you have the injection during the first five days of your cycle, you will be immediately protected against becoming pregnant.
If you have the injection on any other day of your cycle, you will not be protected against pregnancy for up to seven days. Use condoms or another method of contraception during this time.
After giving birth
You can have the contraceptive injection at any time after you have given birth, if you are not breastfeeding. If you are breastfeeding, the injection will usually be given after six weeks, although it may be given earlier if necessary.
- If you start injections on or before day 21 after giving birth, you will be immediately protected against becoming pregnant.
- If you start injections after day 21, you will need to use additional contraception for the following seven days.
Heavy and irregular bleeding is more likely to occur if you have the contraceptive injection during the first few weeks after giving birth.
It is safe to use contraceptive injections while you are breastfeeding.
After a miscarriage or abortion
You can have the injection immediately after a miscarriage or abortion, and you will be protected against pregnancy straight away. If you have the injection more than five days after a miscarriage or abortion, you'll need to use additional contraception for seven days.
Who can use the injection?
Most women can be given the contraceptive injection. It may not be suitable if you:
- think you might be pregnant
- want to keep having regular periods
- have bleeding in between periods or after sex
- have arterial disease or a history of heart disease or stroke
- have a blood clot in a blood vessel (thrombosis)
- have liver disease
- have migraines
- have breast cancer or have had it in the past
- have diabetes with complications
- have cirrhosis or liver tumours
- are at risk of osteoporosis
Advantages and disadvantages of the injection
The main advantages of the contraceptive injection are:
- each injection lasts for either eight, 12 or 13 weeks
- the injection does not interrupt sex
- the injection is an option if you cannot use oestrogen-based contraception, such as the combined pill, contraceptive patch or vaginal ring
- you do not have to remember to take a pill every day
- the injection is safe to use while you are breastfeeding
- the injection is not affected by other medicines
- the injection may reduce heavy, painful periods and help with premenstrual symptoms for some women
- the injection offers some protection from pelvic inflammatory disease (the mucus from the cervix may stop bacteria entering the womb) and may also give some protection against cancer of the womb
Using the contraceptive injection may have some disadvantages, which you should consider carefully before deciding on the right method of contraception for you. These are as follows:
Disrupted periods
Your periods may change significantly during the first year of using the injection. They will usually become irregular and may be very heavy, or shorter and lighter, or stop altogether. This may settle down after the first year, but may continue as long as the injected progestogen remains in your body.
It can take a while for your periods and natural fertility to return after you stop using the injection. It takes around eight to 12 weeks for injected progestogen to leave the body, but you may have to wait longer for your periods to return to normal if you are trying to get pregnant.
Until you are ovulating regularly each month, it can be difficult to work out when you are at your most fertile. In some cases, it can take three months to a year for your periods to return to normal.
Weight gain
You may put on weight when you use the contraceptive injection, particulaly if you are under 18 years old and are overweight with a BMI (body mass index) of 30 or over.
Other side effects that some women report are:
- headaches
- acne
- tender breasts
- changes in mood
- loss of sex drive
Depo-Provera, oestrogen and bone risk
Using Depo-Provera affects your natural oestrogen levels, which can cause thinning of the bones, but it does not increase your risk of breaking a bone. This isn't a problem for most women, because the bone replaces itself when you stop the injection, and it doesn't appear to cause any long-term problems.
Thinning of the bones may be a problem for women who already have an increased risk of developing osteoporosis (for example, because they have low oestrogen, or a family history of osteoporosis). It may also be a concern for women under 18, because the body is still making bone at this age. Women under 18 may use Depo-Provera, but only after careful evaluation by a doctor.
Will other medicines affect the injection?
No – the contraceptive injection is not affected by other medication.
Risks
There is a small risk of infection at the site of the injection. In very rare cases, some people may have an allergic reaction to the injection.
Where you can get it
Most types of contraception are available free in the UK. Contraception is free to all women and men through the NHS. You can get contraception at:
- most GP surgeries – talk to your GP or practice nurse
- community contraception clinics
- some genitourinary medicine (GUM) clinics
- sexual health clinics – they also offer contraceptive and STI testing services
- some young people’s services (call 0300 123 7123 for more information)
Find your nearest sexual health clinic by searching your postcode or town.
Contraception services are free and confidential, including for people under the age of 16.
If you're under 16 and want contraception, the doctor, nurse or pharmacist won't tell your parents (or carer) as long as they believe you fully understand the information you're given, and your decisions. Doctors and nurses work under strict guidelines when dealing with people under 16.
They'll encourage you to consider telling your parents, but they won't make you. The only time that a professional might want to tell someone else is if they believe you're at risk of harm, such as abuse. The risk would need to be serious, and they would usually discuss this with you first.
Find out more about the medicines used in the contraceptive injection.
Medicines for Contraceptive implants and injections
 Over-the-counter medicine. Medicine with this icon can be bought without a prescription.
Over-the-counter medicine. Medicine with this icon can be bought without a prescription.
E-Etonogestrel-(a generic version of )
I-
N-
-Noristerat (a brand of )
Contraceptive Implant
A woman can get pregnant if a man’s sperm reaches one of her eggs (ova). Contraception tries to stop this happening by keeping the egg and sperm apart or by stopping egg production. One method is the implant.
- At a glance: facts about the contraceptive implant
- How it works
- Who can use it
- Advantages and disadvantages
- Risks
- Where you can get it
The contraceptive implant is a small flexible tube about 40mm long that's inserted under the skin of your upper arm. It's inserted by a trained professional, such as a doctor, and lasts for three years.
The implant stops the release of an egg from the ovary by slowly releasing progestogen into your body. Progestogen thickens the cervical mucus and thins the womb lining. This makes it harder for sperm to move through your cervix, and less likely for your womb to accept a fertilised egg.
At a glance: the implant
- If implanted correctly, it's more than 99% effective. Fewer than one woman in 1,000 who use the implant as contraception will get pregnant in one year.
- It's very useful for women who know they don't want to get pregnant for a while. Once the implant is in place, you don't have to think about contraception for three years.
- It can be useful for women who can't use contraception that contains oestrogen.
- It's very useful for women who find it difficult to take a pill at the same time every day.
- If you have side effects, the implant can be taken out. You can have the implant removed at any time, and your natural fertility will return very quickly.
- When it's first put in, you may feel some bruising, tenderness or swelling around the implant.
- In the first year after the implant is fitted, your periods may become irregular, lighter, heavier or longer. This usually settles down after the first year.
- A common side effect of the implant is that your periods stop (amenorrhoea). It's not harmful, but you may want to consider this before deciding to have an implant.
- Some medications can make the implant less effective, and additional contraceptive precautions need to be followed when you are taking these medications (see Will other medicines affect the implant?).
- The implant does not protect against sexually transmitted infections (STIs). By using condoms as well as the implant, you'll help to protect yourself against STIs.
How the implant works
The implant steadily releases the hormone progestogen into your bloodstream. Progestogen is similar to the natural hormone progesterone, which is released by a woman's ovaries during her period.
The continuous release of progestogen:
- stops a woman releasing an egg every month (ovulation)
- thickens the mucus from the cervix (entrance to the womb), making it difficult for sperm to pass through to the womb and reach an unfertilised egg
- makes the lining of the womb thinner so that it is unable to support a fertilised egg
- most GP surgeries
- community contraception clinics
- some GUM clinics
- sexual health clinics
- some young people's services
Find a clinic near you
The implant can be put in at any time during your menstrual cycle, as long as you and your doctor are reasonably sure you are not pregnant. In the UK, Nexplanon is the main contraceptive implant currently in use. Implants inserted before October 2010 were called Implanon. Since October 2010, insertion of Implanon has decreased as stocks are used up, and Nexplanon has become the most commonly used implant.
Both types of implant work in the same way, but Nexplanon is designed to reduce the risk of insertion errors and is visible on an X-ray or CT (computerised tomography) scan. There is no need for existing Implanon users to have their implant removed and replaced by Nexplanon ahead of its usual replacement time.
Nexplanon is a small, thin, flexible tube about 4cm long. It is implanted under the skin of your upper arm by a doctor or nurse. A local anaesthetic is used to numb the area. The small wound made in your arm is closed with a dressing and does not need stitches.
Nexplanon works for up to three years before it needs to be replaced. You can continue to use it until you reach the menopause, when a woman’s monthly periods stop (at around 52 years of age). The implant can be removed at any time by a specially trained doctor or nurse. It only takes a few minutes to remove, using a local anaesthetic.
As soon as the implant has been removed, you will no longer be protected against pregnancy.
When it starts to work
If the implant is fitted during the first five days of your menstrual cycle, you will be immediately protected against becoming pregnant. If it is fitted on any other day of your menstrual cycle, you will not be protected against pregnancy for up to seven days, and should use another method, such as condoms.
After giving birth
You can have the contraceptive implant fitted after you have given birth, usually after three weeks.
- If it is fitted on or before day 21 after the birth, you will be immediately protected against becoming pregnant.
- If it is fitted after day 21, you will need to use additional contraception, such as condoms, for the following seven days.
It is safe to use the implant while you are breastfeeding.
After a miscarriage or abortion
The implant can be fitted immediately after a miscarriage or an abortion, and you will be protected against pregnancy straight away.
Who can use the implant
Most women can be fitted with the contraceptive implant. It may not be suitable if you:
- think you might be pregnant
- want to keep having regular periods
- have bleeding in between periods or after sex
- have arterial disease or a history of heart disease or stroke
- have a blood clot in a blood vessel (thrombosis)
- have liver disease
- have migraines
- have breast cancer or have had it in the past
- have diabetes with complications
- have cirrhosis or liver tumours
- are at risk of osteoporosis
Advantages and disadvantages of the implant
The main advantages of the contraceptive implant are:
- it works for three years
- the implant does not interrupt sex
- it is an option if you cannot use oestrogen-based contraception, such as the combined contraceptive pill, contraceptive patch or vaginal ring
- you do not have to remember to take a pill every day
- the implant is safe to use while you are breastfeeding
- your fertility should return to normal as soon as the implant is removed
- implants offer some protection against pelvic inflammatory disease (the mucus from the cervix may stop bacteria entering the womb) and may also give some protection against cancer of the womb
- the implant may reduce heavy periods or painful periods after the first year of use
- after the contraceptive implant has been inserted, you should be able to carry out normal activities
Using a contraceptive implant may have some disadvantages, which you should consider carefully before deciding on the right method of contraception for you. These include:
Disrupted periods
Your periods may change significantly while using a contraceptive implant. Around 20% of women using the implant will have no bleeding, and almost 50% will have infrequent or prolonged bleeding. Bleeding patterns are likely to remain irregular, although they may settle down after the first year.
Although these changes are not harmful, they may not be acceptable for some women. Your GP may be able to help by providing additional medication if you have prolonged bleeding.
Other side effects that some women report are:
- headaches
- acne
- nausea
- breast tenderness
- changes in mood
- loss of sex drive
These side effects usually stop after the first few months. If you have prolonged or severe headaches or other side effects, tell your doctor.
Some women put on weight while using the implant, but there is no evidence to show that the implant causes weight gain.
Will other medicines affect the implant?
Some medicines can reduce the implant's effectiveness. These include:
- medication for HIV
- medication for epilepsy
- complementary remedies, such as St John's Wort
- an antibiotic called rifabutin (which can be used to treat tuberculosis)
- an antibiotic called rifampicin (which can be used to treat several conditions, including tuberculosis and meningitis)
These are called enzyme-inducing drugs. If you are using these medicines for a short while (for example, rifampicin to protect against meningitis), it is recommended that you use additional contraception during the course of treatment and for 28 days afterwards. The additional contraception could be condoms, or a single dose of the contraceptive injection. The implant can remain in place if you have the injection.
Women taking enzyme-inducing drugs in the long term may wish to consider using a method of contraception that isn't affected by their medication.
Always tell your doctor that you are using an implant if you are prescribed any medicines. Ask your doctor or nurse for more details about the implant and other medication.
Risks of the implant
In rare cases, the area of skin where the implant has been fitted can become infected. If this happens, the area will be cleaned and may be treated with antibiotics.
Where you can get the contraceptive implant
Most types of contraception are available for free in the UK. Contraception is free to all women and men through the NHS. Places where you can get contraception include:
- most GP surgeries – talk to your GP or practice nurse
- community contraception clinics
- some genitourinary medicine (GUM) clinics
- sexual health clinics – they also offer contraceptive and STI testing services
- some young people’s services (call 0300 123 7123 for more information)
Find your nearest sexual health clinic by searching by postcode or town.
Contraception services are free and confidential, including for people under the age of 16.
If you're under 16 and want contraception, the doctor, nurse or pharmacist won't tell your parents (or carer) as long as they believe you fully understand the information you're given, and your decisions. Doctors and nurses work under strict guidelines when dealing with people under 16. They'll encourage you to consider telling your parents, but they won't make you. The only time that a professional might want to tell someone else is if they believe you're at risk of harm, such as abuse. The risk would need to be serious, and they would usually discuss this with you first.
Page last reviewed: 31/12/2014
Next review due: 31/12/2016
IUS (intrauterine system)
A woman can get pregnant if a man’s sperm reaches one of her eggs (ova). Contraception tries to stop this happening by keeping the egg and sperm apart or by stopping egg production. One method of contraception is the IUS, or intrauterine system (sometimes called the hormonal coil).
- At a glance: facts about the IUS
- How the IUS works
- Who can use the IUS
- Advantages and disadvantages of the IUS
- Risks of the IUS
- Where to get the IUS
An IUS is a small, T-shaped plastic device that is inserted into your womb (uterus) by a specially trained doctor or nurse.
The IUS releases a progestogen hormone into the womb. This thickens the mucus from your cervix, making it difficult for sperm to move through and reach an egg. It also thins the womb lining so that it's less likely to accept a fertilised egg. It may also stop ovulation (the release of an egg) in some women.
The IUS is a long-acting reversible contraceptive (LARC) method. It works for five years or three years, depending on the type, so you don't have to think about contraception every day or each time you have sex. Two brands of IUS are used in the UK – Mirena and Jaydess.
You can use an IUS whether or not you've had children.
At a glance: facts about the IUS
- It's more than 99% effective.Less than one in every 100 women who use Mirena will get pregnant in five years, and less than one in 100 who use Jaydess will get pregnant in three years.
- It can be taken out at any time by a specially trained doctor or nurse and your fertility quickly returns to normal.
- The IUS can make your periods lighter, shorter or stop altogether, so it may help women who have heavy periods or painful periods. Jaydess is less likely than Mirena to make your periods stop altogether.
- It can be used by women who can't use combined contraception (such as the combined pill) – for example, those who have migraines.
- Once the IUS is in place, you don't have to think about contraception every day or each time you have sex.
- Some women may experience mood swings, skin problems or breast tenderness.
- There's a small risk of getting an infection after it's inserted.
- It can be uncomfortable when the IUS is put in, although painkillers can help with this.
- The IUS can be fitted at any time during your monthly menstrual cycle, as long as you're definitely not pregnant. Ideally, it should be fitted within seven days of the start of your period, because this will protect against pregnancy straight away. You should use condoms for seven days if the IUS is fitted at any other time.
- The IUS does not protect against sexually transmitted infections (STIs). By using condoms as well as the IUS, you'll help to protect yourself against STIs.
How the IUS works
How it prevents pregnancy
Having an IUS fitted
How to tell whether an IUS is still in place
Removing an IUS
How it prevents pregnancy
The IUS is similar to the IUD (intrauterine device), but works in a slightly different way. Rather than releasing copper like the IUD, the IUS releases a progestogen hormone, which is similar to the natural hormone progesterone that's produced in a woman's ovaries.
Progestogen thickens the mucus from the cervix (opening of the womb), making it harder for sperm to move through it and reach an egg. It also causes the womb lining to become thinner and less likely to accept a fertilised egg. In some women, the IUS also stops the ovaries from releasing an egg (ovulation), but most women will continue to ovulate.
If you're 45 or older when you have the IUS fitted, it can be left until you reach menopause or you no longer need contraception.
Having an IUS fitted
- most GP surgeries
- community contraception clinics
- some GUM clinics
- sexual health clinics
- some young people's services
Find a clinic near you
An IUS can be fitted at any stage of your menstrual cycle, as long as you are not pregnant. If it's fitted in the first seven days of your cycle, you will be protected against pregnancy straight away. If it's fitted at any other time, you need to use another method of contraception (such as condoms) for seven days after it's fitted.
Before you have an IUS fitted, you will have an internal examination to determine the size and position of your womb. This is to make sure that the IUS can be positioned in the correct place.
You may also be tested for any existing infections, such as STIs. It is best to do this before an IUS is fitted so that any infections can be treated. You may be given antibiotics at the same time as an IUS is fitted.
It takes about 15 to 20 minutes to insert an IUS:
- the vagina is held open, like it is during a cervical screening (smear) test
- the IUS is inserted through the cervix and into the womb
The fitting process can be uncomfortable or painful for some women, and you may also experience cramps afterwards.
You can ask for a local anaesthetic or painkillers before having the IUS fitted. Discuss this with your GP or nurse beforehand. An anaesthetic injection itself can be painful, so many women have the procedure without one.
Once an IUS is fitted, it will need to be checked by a doctor after three to six weeks to make sure everything is fine. Speak to your GP or clinician if you have any problems after this initial check or if you want the IUS removed.
Also speak to your GP if you or your partner are at risk of getting an STI, as this can lead to infection in the pelvis.
See your GP or go back to the clinic if you:
- have pain in your lower abdomen
- have a high temperature
- have smelly discharge
This may mean you have an infection.
How to tell if an IUS is still in place
An IUS has two thin threads that hang down a little way from your womb into the top of your vagina. The GP or clinician that fits your IUS will teach you how to feel for these threads and check that the IUS is still in place.
Check your IUS is in place a few times in the first month and then after each period at regular intervals.
It is highly unlikely that your IUS will come out, but if you can't feel the threads or if you think the IUS has moved, you may not be fully protected against pregnancy. See your doctor or nurse straight away and use extra contraception, such as condoms, until your IUS has been checked. If you've had sex recently, you may need to use emergency contraception.
Your partner shouldn't be able to feel your IUS during sex. If he can feel the threads, get your GP or clinician to check that your IUS is in place. They may be able to cut the threads a little. If you feel any pain during sex, go for a check-up with your GP or clinician.
Removing an IUS
Your IUS can be removed at any time by a trained doctor or nurse.
If you're not going to have another IUS put in and you don't want to become pregnant, use another contraceptive method (such as condoms) for seven days before you have the IUS removed. Sperm can live for seven days in the body and could fertilise an egg once the IUS is removed. As soon as an IUS is taken out, your normal fertility should return.
Who can use an IUS
Most women can use an IUS, including women who have never been pregnant and those who are HIV positive. Your GP or clinician will ask about your medical history to check if an IUS is the most suitable form of contraception for you.
Your family and medical history will determine whether or not you can use an IUS. For example, this method of contraception may not be suitable for you if you have:
- breast cancer, or have had it in the past five years
- cervical cancer
- liver disease
- unexplained vaginal bleeding between periods or after sex
- arterial disease or history of serious heart disease or stroke
- an untreated STI or pelvic infection
- problems with your womb or cervix
An IUS may not be suitable for women who have untreated STIs. A doctor will usually give you a check-up to make sure you don't have any existing infections.
Using an IUS after giving birth
An IUS can usually be fitted four to six weeks after giving birth (vaginal or caesarean). You'll need to use alternative contraception from three weeks (21 days) after the birth until the IUS is put in. In some cases, an IUS can be fitted within 48 hours of giving birth. It is safe to use an IUS when you're breastfeeding, and it won't affect your milk supply.
Using an IUS after a miscarriage or abortion
An IUS can be fitted by an experienced doctor or nurse straight after an abortion or miscarriage, as long as you were pregnant for less than 24 weeks. If you were pregnant for more than 24 weeks, you may have to wait a few weeks before an IUS can be fitted.
Advantages and disadvantages of the IUS
Although an IUS is an effective method of contraception, there are several things to consider before having an IUS fitted.
Advantages of the IUS
- It works for five years (Mirena) or three years (Jaydess).
- It's one of the most effective forms of contraception available in the UK.
- It doesn't interrupt sex.
- An IUS may be useful if you have heavy or painful periods because your periods usually become much lighter and shorter, and sometimes less painful – they may stop completely after the first year of use.
- It can be used safely if you're breastfeeding.
- It's not affected by other medicines.
- It may be a good option if you can't take the hormone oestrogen, which is used in the combined contraceptive pill.
- Your fertility will return to normal when the IUS is removed.
There's no evidence that an IUS will affect your weight or that having an IUS fitted will increase the risk of cervical cancer, cancer of the uterus or ovarian cancer. Some women experience changes in mood and libido, but these changes are very small.
Disadvantages of the IUS
- Some women won't be happy with the way that their periods may change. For example, periods may become lighter and more irregular or, in some cases, stop completely. Your periods are more likely to stop completely with Mirena than with Jaydess.
- Irregular bleeding and spotting are common in the first six months after having an IUS fitted. This is not harmful and usually decreases with time.
- Some women experience headaches, acne and breast tenderness after having the IUS fitted.
- An uncommon side effect of the IUS is the appearance of small fluid-filled cysts on the ovaries – these usually disappear without treatment.
- An IUS doesn't protect you against STIs, so you may also have to use condoms when having sex. If you get an STI while you have an IUS fitted, it could lead to pelvic infection if it's not treated.
- Most women who stop using an IUS do so because of vaginal bleeding and pain, although this is uncommon. Hormonal problems can also occur, but these are even less common.
Risks of the IUS
Complications caused by an IUS are rare and usually happen in the first six months after it has been fitted. These include:
Damage to the womb
In rare cases (fewer than one in 1,000 insertions) an IUS can perforate (make a hole in) the womb or neck of the womb (cervix) when it is put in. This can cause pain in the lower abdomen, but doesn't usually cause any other symptoms. If the doctor or nurse fitting your IUS is experienced, the risk of perforation is extremely low.
If perforation occurs, you may need surgery to remove the IUS. Contact your GP straight away if you feel a lot of pain after having an IUS fitted. Perforations should be treated immediately.
Pelvic infections
Pelvic infections may occur in the first 20 days after the IUS has been inserted.
The risk of infection from an IUS is extremely small (fewer than one in 100 women who are at low risk of STIs will get an infection). A GP or clinician will usually recommend an internal examination before fitting an IUS to be sure that there are no existing infections.
Rejection
Occasionally, the IUS is rejected (expelled) by the womb or it can move (this is called displacement). This is not common and is more likely to happen soon after it has been fitted. Your doctor or nurse will teach you how to check that your IUS is in place.
Ectopic pregnancy
If the IUS fails and you become pregnant, your IUS should be removed as soon as possible if you are continuing with the pregnancy. There's a small increased risk of ectopic pregnancy if a woman becomes pregnant while using an IUS.
Where to get the IUS
Most types of contraception are available for free in the UK. Contraception is free to all women and men through the NHS. Places where you can get contraception include:
- most GP surgeries – talk to your GP or practice nurse
- community contraception clinics
- some genitourinary medicine (GUM) clinics
- sexual health clinics – they also offer contraceptive and STI testing services
- some young people’s services (call 0300 123 7123 for more information)
Find sexual health services near you, including contraception clinics.
Contraception services are free and confidential, including for people under the age of 16.
If you're under 16 and want contraception, the doctor, nurse or pharmacist won't tell your parents or carer as long as they believe you fully understand the information you're given and your decisions. Doctors and nurses work under strict guidelines when dealing with people under 16.
They'll encourage you to consider telling your parents, but they won't make you. The only time that a professional might want to tell someone else is if they believe you're at risk of harm, such as abuse. The risk would need to be serious, and they would usually discuss this with you first.
IUD (intrauterine device)
A woman can get pregnant if a man’s sperm reaches one of her eggs (ova). Contraception tries to stop this by keeping the egg and sperm apart or by stopping eggs being produced. One method of contraception is the intrauterine device, or IUD (sometimes called a coil).
- At a glance: facts about the IUD
- How the IUD works
- Who can use the IUD
- Advantages and disadvantages of the IUD
- Risks of the IUD
- Where you can get an IUD
An IUD is a small T-shaped plastic and copper device that’s inserted into your womb (uterus) by a specially trained doctor or nurse.
The IUD works by stopping the sperm and egg from surviving in the womb or fallopian tubes. It may also prevent a fertilised egg from implanting in the womb.
The IUD is a long-acting reversible contraceptive (LARC) method. This means that once it's in place, you don't have to think about it each day or each time you have sex. There are several types and sizes of IUD.
You can use an IUD whether or not you've had children.
At a glance: facts about the IUD
- There are different types of IUD, some with more copper than others. IUDs with more copper are more than 99% effective. This means that fewer than one in 100 women who use an IUD will get pregnant in one year. IUDs with less copper will be less effective.
- An IUD works as soon as it's put in, and lasts for five to 10 years, depending on the type.
- It can be put in at any time during your menstrual cycle, as long as you're not pregnant.
- It can be removed at any time by a specially trained doctor or nurse and you'll quickly return to normal levels of fertility.
- Changes to your periods (for example, being heavier, longer or more painful) are common in the first three to six months after an IUD is put in, but they're likely to settle down after this. You might get spotting or bleeding between periods.
- There's a very small chance of infection within 20 days of the IUD being fitted.
- There's a risk that your body may expel the IUD.
- If you get pregnant, there's an increased risk of ectopic pregnancy (when the egg implants outside the womb). But because you're unlikely to get pregnant, the overall risk of ectopic pregnancy is lower than in women who don't use contraception.
- Having the IUD put in can be uncomfortable. Ask the doctor or nurse about pain relief.
- An IUD may not be suitable for you if you've had previous pelvic infections.
- The IUD does not protect against sexually transmitted infections (STIs). By using condoms as well as the IUD, you'll help to protect yourself against STIs.
How the IUD works
How it prevents pregnancy
Having an IUD fitted
How to tell whether an IUD is still in place
Removing an IUD
How it prevents pregnancy
The IUD is similar to the IUS (intrauterine system) but works in a different way. Instead of releasing the hormone progestogen like the IUS, the IUD releases copper. Copper changes the make-up of the fluids in the womb and fallopian tubes, stopping sperm surviving there. IUDs may also stop fertilised eggs from implanting in the womb.
There are types and sizes of IUD to suit different women. IUDs need to be fitted by a trained doctor or nurse at your GP surgery, local contraception clinic or sexual health clinic.
An IUD can stay in the womb for five to 10 years, depending on the type. If you're 40 or over when you have an IUD fitted, it can be left in until you reach the menopause or until you no longer need contraception.
Having an IUD fitted
An IUD can be fitted at any time during your menstrual cycle, as long as you are not pregnant. You'll be protected against pregnancy straight away.
Before you have an IUD fitted, you will have an internal examination to find out the size and position of your womb. This is to make sure that the IUD can be put in the correct place.
- most GP surgeries
- community contraception clinics
- some GUM clinics
- sexual health clinics
- some young people's services
Find a clinic near you
You may also be tested for infections, such as STIs. It's best to do this before an IUD is fitted so that you can have treatment (if you need it) before the IUD is put in. Sometimes, you may be given antibiotics at the same time as the IUD is fitted.
It takes about 15 to 20 minutes to insert an IUD. The vagina is held open, like it is during a cervical screening (smear) test, and the IUD is inserted through the cervix and into the womb.
The fitting process can be uncomfortable and sometimes painful. You may get cramps afterwards. You can ask for a local anaesthetic or painkillers before having the IUD fitted. An anaesthetic injection itself can be painful, so many women have the procedure without.
You may get pain and bleeding for a few days after having an IUD fitted. Discuss this with your GP or nurse beforehand.
The IUD needs to be checked by a doctor after three to six weeks. Speak to your doctor or nurse if you have any problems before or after this first check or if you want the IUD removed.
Speak to your doctor or nurse if you or your partner are at risk of getting an STI. This is because STIs can lead to an infection in the pelvis.
See your GP or go back to the clinic where your IUD was fitted as soon as you can if you:
- have pain in your lower abdomen
- have a high temperature
- have a smelly discharge
These may mean you have an infection.
How to tell whether an IUD is still in place
An IUD has two thin threads that hang down a little way from your womb into the top of your vagina. The doctor or nurse who fits your IUD will teach you how to feel for these threads and check that it is still in place.
Check your IUD is in place a few times in the first month, and then after each period or at regular intervals.
It's very unlikely that your IUD will come out, but if you can't feel the threads, or if you think the IUD has moved, you may not be fully protected against getting pregnant. See your doctor or nurse straight away and use an extra method of contraception, such as condoms, until your IUD has been checked. If you've had sex recently, you may need to use emergency contraception.
Your partner shouldn't be able to feel your IUD during sex. If he can feel the threads, get your doctor or nurse to check that your IUD is in place. They may be able to cut the threads to a shorter length. If you feel any pain during sex, go for a check-up.
Removing an IUD
An IUD can be removed at any time by a trained doctor or nurse.
If you're not going to have another IUD put in and you don't want to get pregnant, use another method (such as condoms) for seven days before you have the IUD removed. This is to stop sperm getting into your body. Sperm can live for up to seven days in the body and could make you pregnant once the IUD is removed.
As soon as an IUD is taken out, your normal fertility should return.
Who can use an IUD
Most women can use an IUD. This includes women who have never been pregnant and those who are HIV positive. Your doctor or nurse will ask about your medical history to check if an IUD is the most suitable form of contraception for you.
You should not use an IUD if you have:
- an untreated STI or a pelvic infection
- problems with your womb or cervix
- any unexplained bleeding from your vagina – for example, between periods or after sex
Women who have had an ectopic pregnancy or recent abortion, or who have an artificial heart valve, must consult their GP or clinician before having an IUD fitted.
You should not be fitted with an IUD if there's a chance that you are already pregnant or if you or your partner are at risk of catching STIs. If you or your partner are unsure, go to your GP or a sexual health clinic to be tested.
Using an IUD after giving birth
An IUD can usually be fitted four to six weeks after giving birth (vaginal or caesarean). You'll need to use alternative contraception from three weeks (21 days) after the birth until the IUD is fitted. In some cases, an IUD can be fitted within 48 hours of giving birth. An IUD is safe to use when you're breastfeeding and it won't affect your milk supply.
Using an IUD after a miscarriage or abortion
An IUD can be fitted straight away or within 48 hours after an abortion or miscarriage by an experienced doctor or nurse, as long as you were pregnant for less than 24 weeks. If you were pregnant for more than 24 weeks, you may have to wait a few weeks before having an IUD fitted.
Advantages and disadvantages of the IUD
Although an IUD is an effective method of contraception, there are some things to consider before having one fitted.
Advantages of the IUD
- Most women can use an IUD, including women who have never been pregnant.
- Once an IUD is fitted, it works straight away and lasts for up to 10 years or until it's removed.
- It doesn't interrupt sex.
- It can be used if you're breastfeeding.
- Your normal fertility returns as soon as the IUD is taken out
- It's not affected by other medicines.
There's no evidence that having an IUD fitted will increase the risk of cancer of the cervix, endometrial cancer (cancer of the lining of the womb) or ovarian cancer. Some women experience changes in mood and libido, but these changes are very small. There is no evidence that the IUD affects weight.
Disadvantages of the IUD
- Your periods may become heavier, longer or more painful, though this may improve after a few months.
- An IUD doesn't protect against STIs, so you may have to use condoms as well. If you get an STI while you have an IUD, it could lead to a pelvic infection if not treated.
- The most common reasons that women stop using an IUD are vaginal bleeding and pain.
Risks of the IUD
Complications after having an IUD fitted are rare. Most will appear within the first year after fitting.
Damage to the womb
In fewer than one in 1,000 cases, an IUD can perforate (make a hole in) the womb or neck of the womb (cervix) when it's put in. This can cause pain in the lower abdomen, but doesn't usually cause any other symptoms. If the doctor or nurse fitting your IUD is experienced, the risk of this is very low.
If perforation occurs, you may need surgery to remove the IUD. Contact your GP straight away if you feel a lot of pain after having an IUD fitted as perforations should be treated immediately.
Pelvic infections
Pelvic infections can occur in the first 20 days after the IUD is fitted. The risk of infection is very small. Fewer than one in 100 women who are at low risk of STIs will get a pelvic infection.
Rejection
Occasionally, the IUD is rejected (expelled) by the womb or can move (this is called displacement). This is more likely to happen soon after it has been fitted, although this is uncommon. Your doctor or nurse will teach you how to check that your IUD is in place.
Ectopic pregnancy
If the IUD fails and you become pregnant, your IUD should be removed as soon as possible if you're going to continue with the pregnancy. There's a small increased risk of ectopic pregnancy if a woman becomes pregnant while using an IUD.
Where to get an IUD
Most types of contraception are available free in the UK. Contraception is free to all women and men through the NHS. Places where you can get contraception include:
- most GP surgeries – talk to your GP or practice nurse
- community contraception clinics
- some genitourinary medicine (GUM) clinics
- sexual health clinics – these offer contraceptive and STI testing services
- some young people’s services (call the sexual health line on 0300 123 7123 for details)
Find your nearest sexual health clinic by searching your postcode or town.
If you're under 16 and want contraception, the doctor, nurse or pharmacists won't tell your parents or carer, as long as they believe you fully understand the information you're given, and your decisions.
Doctors and nurses work under strict guidelines when dealing with people under 16. They'll encourage you to consider telling your parents, but they won't make you. The only time that a professional might want to tell someone else is if they believe you're at risk of harm, such as abuse. The risk would need to be serious, and they would usually discuss this with you first.
Source: NHS Choices, UK
back-to-top
Sexually transmitted infections (STI) fact sheet
- What is a sexually transmitted infection (STI)?
- How many people have STIs and who is infected?
- How do you get an STI?
- Can STIs cause health problems?
- What are the symptoms of STIs?
- How do you get tested for STIs?
- Who needs to get tested for STIs?
- How are STIs treated?
- What can I do to keep from getting an STI?
- How do STIs affect pregnant women and their babies?
- What can pregnant women do to prevent problems from STIs?
- Do STIs affect breastfeeding?
- Is there any research being done on STIs?
- For more information
- More information on sexually transmitted infections (STI)
What is a sexually transmitted infection (STI)?
It is an infection passed from person to person through intimate sexual contact. STIs are also called sexually transmitted diseases, or STDs.
How many people have STIs and who is infected?
In the United States about 19 million new infections are thought to occur each year. These infections affect men and women of all backgrounds and economic levels. But almost half of new infections are among young people ages 15 to 24. Women are also severely affected by STIs. They have more frequent and more serious health problems from STIs than men. African-American women have especially high rates of infection.
How do you get an STI?
You can get an STI by having intimate sexual contact with someone who already has the infection. You can’t tell if a person is infected because many STIs have no symptoms. But STIs can still be passed from person to person even if there are no symptoms. STIs are spread during vaginal, anal, or oral sex or during genital touching. So it’s possible to get some STIs without having intercourse. Not all STIs are spread the same way.
Can STIs cause health problems?
Yes. Each STI causes different health problems. But overall, untreated STIs can cause cancer, pelvic inflammatory disease, infertility, pregnancy problems, widespread infection to other parts of the body, organ damage, and even death.
Having an STI also can put you at greater risk of getting HIV. For one, not stopping risky sexual behavior can lead to infection with other STIs, including HIV. Also, infection with some STIs makes it easier for you to get HIV if you are exposed.
What are the symptoms of STIs?
Many STIs have only mild or no symptoms at all. When symptoms do develop, they often are mistaken for something else, such as urinary tract infection or yeast infection. This is why screening for STIs is so important. The STIs listed here are among the most common or harmful to women.
Symptoms of sexually transmitted infections
| STI |
Symptoms |
| Bacterial vaginosis (BV) |
Most women have no symptoms. Women with symptoms may have:
- Vaginal itching
- Pain when urinating
- Discharge with a fishy odor
|
| Chlamydia |
Most women have no symptoms. Women with symptoms may have:
- Abnormal vaginal discharge
- Burning when urinating
- Bleeding between periods
Infections that are not treated, even if there are no symptoms, can lead to:
- Lower abdominal pain
- Low back pain
- Nausea
- Fever
- Pain during sex
|
| Genital herpes |
Some people may have no symptoms. During an “outbreak,” the symptoms are clear:
- Small red bumps, blisters, or open sores where the virus entered the body, such as on the penis, vagina, or mouth
- Vaginal discharge
- Fever
- Headache
- Muscle aches
- Pain when urinating
- Itching, burning, or swollen glands in genital area
- Pain in legs, buttocks, or genital area
Symptoms may go away and then come back. Sores heal after 2 to 4 weeks.
|
| Gonorrhea |
Symptoms are often mild, but most women have no symptoms. If symptoms are present, they most often appear within 10 days of becoming infected. Symptoms are:
- Pain or burning when urinating
- Yellowish and sometimes bloody vaginal discharge
- Bleeding between periods
- Pain during sex
- Heavy bleeding during periods
Infection that occurs in the throat, eye, or anus also might have symptoms in these parts of the body.
|
| Hepatitis B |
Some women have no symptoms. Women with symptoms may have:
- Low-grade fever
- Headache and muscle aches
- Tiredness
- Loss of appetite
- Upset stomach or vomiting
- Diarrhea
- Dark-colored urine and pale bowel movements
- Stomach pain
- Skin and whites of eyes turning yellow
|
| HIV/AIDS |
Some women may have no symptoms for 10 years or more. About half of people with HIV get flu-like symptoms about 3 to 6 weeks after becoming infected. Symptoms people can have for months or even years before the onset of AIDS include:
- Fevers and night sweats
- Feeling very tired
- Quick weight loss
- Headache
- Enlarged lymph nodes
- Diarrhea, vomiting, and upset stomach
- Mouth, genital, or anal sores
- Dry cough
- Rash or flaky skin
- Short-term memory loss
Women also might have these signs of HIV:
- Vaginal yeast infections and other vaginal infections, including STIs
- Pelvic inflammatory disease (PID) that does not get better with treatment
- Menstrual cycle changes
|
| Human papillomavirus (HPV) |
Some women have no symptoms. Women with symptoms may have:
- Visible warts in the genital area, including the thighs. Warts can be raised or flat, alone or in groups, small or large, and sometimes they are cauliflower-shaped.
- Growths on the cervix and vagina that are often invisible.
|
Pubic lice
(sometimes called "crabs") |
Symptoms include:
- Itching in the genital area
- Finding lice or lice eggs
|
| Syphilis |
Syphilis progresses in stages. Symptoms of the primary stage are:
- A single, painless sore appearing 10 to 90 days after infection. It can appear in the genital area, mouth, or other parts of the body. The sore goes away on its own.
If the infection is not treated, it moves to the secondary stage. This stage starts 3 to 6 weeks after the sore appears. Symptoms of the secondary stage are:
- Skin rash with rough, red or reddish-brown spots on the hands and feet that usually does not itch and clears on its own
- Fever
- Sore throat and swollen glands
- Patchy hair loss
- Headaches and muscle aches
- Weight loss
- Tiredness
In the latent stage, symptoms go away, but can come back. Without treatment, the infection may or may not move to the late stage. In the late stage, symptoms are related to damage to internal organs, such as the brain, nerves, eyes, heart, blood vessels, liver, bones, and joints. Some people may die.
|
Trichomoniasis
(sometimes called "trich") |
Many women do not have symptoms. Symptoms usually appear 5 to 28 days after exposure and can include:
- Yellow, green, or gray vaginal discharge (often foamy) with a strong odor
- Discomfort during sex and when urinating
- Itching or discomfort in the genital area
- Lower abdominal pain (rarely)
|
How do you get tested for STIs?
Tests for reproductive health
Bring our Tests for Reproductive Health (PDF, 306 KB) to your next checkup.
There is no one test for all STIs. Ask your doctor about getting tested for STIs. She or he can tell you what test(s) you might need and how it is done. Testing for STIs is also called STI screening. Testing (or screening) for STIs can involve:
- Pelvic and physical exam — Your doctor can look for signs of infection, such as warts, rashes, discharge.
- Blood sample
- Urine sample
- Fluid or tissue sample — A swab is used to collect a sample that can be looked at under a microscope or sent to a lab for testing.
Sexually transmitted infections testing site
Find an STI testing site near you.
These methods are used for many kinds of tests. So if you have a pelvic exam and Pap test, for example, don’t assume that you have been tested for STIs. Pap testing is mainly used to look for cell changes that could be cancer or precancer. Although a Pap test sample also can be used to perform tests for HPV, doing so isn’t routine. And a Pap test does not test for other STIs. If you want to be tested for STIs, including HPV, you must ask.
You can get tested for STIs at your doctor’s office or a clinic. But not all doctors offer the same tests. So it’s important to discuss your sexual health history to find out what tests you need and where you can go to get tested.
Who needs to get tested for STIs?
Screening tests
- Find out what screening tests you might need
If you are sexually active, talk to your doctor about STI screening. Which tests you might need and how often depend mainly on your sexual history and your partner’s. Talking to your doctor about your sex life might seem too personal to share. But being open and honest is the only way your doctor can help take care of you. Also, don’t assume you don’t need to be tested for STIs if you have sex only with women. Talk to your doctor to find out what tests make sense for you.
How are STIs treated?
The treatment depends on the type of STI. For some STIs, treatment may involve taking medicine or getting a shot. For other STIs that can’t be cured, like herpes, treatment can help to relieve the symptoms.
Only use medicines prescribed or suggested by your doctor. There are products sold over the Internet that falsely claim to prevent or treat STIs, such as herpes, chlamydia, human papillomavirus, and HIV. Some of these drugs claim to work better than the drugs your doctor will give you. But this is not true, and the safety of these products is not known.
What can I do to keep from getting an STI?
You can lower your risk of getting an STI with the following steps. The steps work best when used together. No single strategy can protect you from every single type of STI.
- Don’t have sex. The surest way to keep from getting any STI is to practice abstinence. This means not having vaginal, oral, or anal sex. Keep in mind that some STIs, like genital herpes, can be spread without having intercourse.
- Be faithful. Having a sexual relationship with one partner who has been tested for STIs and is not infected is another way to lower your risk of getting infected. Be faithful to each other. This means you only have sex with each other and no one else.
- Use condoms correctly and every time you have sex. Use condoms for all types of sexual contact, even if intercourse does not take place. Use condoms from the very start to the very end of each sex act, and with every sex partner. A male latex condom offers the best protection. You can use a male polyurethane condom if you or your partner has a latex allergy. For vaginal sex, use a male latex condom or a female condom if your partner won’t wear a condom. For anal sex, use a male latex condom. For oral sex, use a male latex condom. A dental dam might also offer some protection from some STIs.
- Know that some methods of birth control, like birth control pills, shots, implants, or diaphragms, will not protect you from STIs. If you use one of these methods, be sure to also use a condom correctly every time you have sex.
- Talk with your sex partner(s) about STIs and using condoms before having sex. It’s up to you to set the ground rules and to make sure you are protected.
- Don’t assume you’re at low risk for STIs if you have sex only with women. Some common STIs are spread easily by skin-to-skin contact. Also, most women who have sex with women have had sex with men, too. So a woman can get an STI from a male partner and then pass it to a female partner.
- Talk frankly with your doctor and your sex partner(s) about any STIs you or your partner has or has had. Talk about symptoms, such as sores or discharge. Try not to be embarrassed. Your doctor is there to help you with any and all health problems. Also, being open with your doctor and partner will help you protect your health and the health of others.
- Have a yearly pelvic exam. Ask your doctor if you should be tested for STIs and how often you should be retested. Testing for many STIs is simple and often can be done during your checkup. The sooner an STI is found, the easier it is to treat.
- Avoid using drugs or drinking too much alcohol. These activities may lead to risky sexual behavior, such as not wearing a condom.
How do STIs affect pregnant women and their babies?
STIs can cause many of the same health problems in pregnant women as women who are not pregnant. But having an STI also can threaten the pregnancy and unborn baby's health. Having an STI during pregnancy can cause early labor, a woman's water to break early, and infection in the uterus after the birth.
Some STIs can be passed from a pregnant woman to the baby before and during the baby’s birth. Some STIs, like syphilis, cross the placenta and infect the baby while it is in the uterus. Other STIs, like gonorrhea, chlamydia, hepatitis B, and genital herpes, can be passed from the mother to the baby during delivery as the baby passes through the birth canal. HIV can cross the placenta during pregnancy and infect the baby during the birth process.
The harmful effects to babies may include:
- Low birth weight
- Eye infection
- Pneumonia
- Infection in the baby’s blood
- Brain damage
- Lack of coordination in body movements
- Blindness
- Deafness
- Acute hepatitis
- Meningitis
- Chronic liver disease
- Cirrhosis
- Stillbirth
Some of these problems can be prevented if the mother receives routine prenatal care, which includes screening tests for STIs starting early in pregnancy and repeated close to delivery, if needed. Other problems can be treated if the infection is found at birth.
What can pregnant women do to prevent problems from STIs?
Pregnant women should be screened at their first prenatal visit for STIs, including:
- Chlamydia
- Gonorrhea
- Hepatitis B
- HIV
- Syphilis
In addition, some experts recommend that women who have had a premature delivery in the past be screened and treated for bacterial vaginosis (BV) at the first prenatal visit. Even if a woman has been tested for STIs in the past, she should be tested again when she becomes pregnant.
Chlamydia, gonorrhea, syphilis, trichomoniasis, and BV can be treated and cured with antibiotics during pregnancy. Viral STIs, such as genital herpes and HIV, have no cure. But antiviral medication may be appropriate for some pregnant woman with herpes to reduce symptoms. For women who have active genital herpes lesions at the onset of labor, a cesarean delivery (C-section) can lower the risk of passing the infection to the newborn. For women who are HIV positive, taking antiviral medicines during pregnancy can lower the risk of giving HIV to the newborn to less than 2 percent. C-section is also an option for some women with HIV. Women who test negative for hepatitis B may receive the hepatitis B vaccine during pregnancy.
Pregnant women also can take steps to lower their risk of getting an STI during pregnancy.
Do STIs affect breastfeeding?
Did you know?
If you have HIV, do not breastfeed. You can pass the virus to your baby.
Talk with your doctor, nurse, or a lactation consultant about the risk of passing the STI to your baby while breastfeeding. If you have chlamydia or gonorrhea, you can keep breastfeeding. If you have syphilis or herpes, you can keep breastfeeding as long as the sores are covered. Syphilis and herpes are spread through contact with sores and can be dangerous to your newborn. If you have sores on your nipple or areola, stop breastfeeding on that breast. Pump or hand express your milk from that breast until the sore clears. Pumping will help keep up your milk supply and prevent your breast from getting engorged or overly full. You can store your milk to give to your baby in a bottle for another feeding. But if parts of your breast pump that contact the milk also touch the sore(s) while pumping, you should throw the milk away.
If you are being treated for an STI, ask your doctor about the possible effects of the drug on your breastfeeding baby. Most treatments for STIs are safe to use while breastfeeding.
Is there any research being done on STIs?
Yes. Research on STIs is a public health priority. Research is focused on prevention, diagnosis, and treatment.
With prevention, researchers are looking at strategies such as vaccines and topical microbicides (meye-KROH-buh-syds). One large study is testing a herpes vaccine for women. Topical microbicides could play a big role in protecting women from getting STIs. But so far, they have been difficult to design. They are gels or creams that would be put into the vagina to kill or stop the STI before it could infect someone. Researchers are also looking at the reasons some people are at higher risk of STIs, and ways to lower these risks.
Early and fast diagnosis of STIs means treatment can start right away. Early treatment helps to limit the effects of an STI and keep it from spreading to others. Researchers are looking at quick, easy, and better ways to test for STIs, including vaginal swabs women can use to collect a sample for testing. They also are studying the reasons why many STIs have no symptoms, which can delay diagnosis.
Research also is underway to develop new ways to treat STIs. For instance, more and more people are becoming infected with types of gonorrhea that do not respond well to drugs. So scientists are working to develop new antibiotics to treat these drug-resistant types. An example of treatment research success is the life-prolonging effects of new drugs used to treat HIV.
More information on sexually transmitted infections (STI)
For more information about sexually transmitted infections (STI), call womenshealth.gov at 800-994-9662 (TDD: 888-220-5446) or contact the following organizations:
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC, HHS
Phone: 800-232-4636 (TDD: 888-232-6348)
- National HIV and STD Testing Resources, CDC, HHS
Phone: 800-458-5231 or 404-679-3860
- National Institute of Allergy and Infectious Diseases, NIH, HHS
Phone: 866- 284-4107 or 301-496-5717 (TDD: 800-877-8339)
Source: Office on Women's Health, HHS
back-to-top

STD/STS Summaries
What are STDs and sexually transmitted infections(STIs)?
STDs are a group of illnesses that are passed from person to person during sexual intercourse, oral sex, or sex play. These diseases can be caused by bacteria, viruses, yeasts, or parasites and are spread through intimate sexual contact involving the penis, vagina, mouth, or anus. STDs are also called venereal diseases or STIs. Health care professionals prefer the term "infection" rather than "disease," because it is possible for a person to have no symptoms but still carry the bacterium or virus and require treatment. Scientists have identified more than 20 different STDs/STIs.
How many people are affected by or at risk for a sexually transmitted disease or sexually transmitted infection (STD/STI)?
Anyone who has had or is having sexual intercourse or oral sex, or who has participated or is participating in sex play, is at risk for acquiring an STD/STI. Fortunately, it is possible for a person to decrease his or her risk by having protected sex and knowing his or her STD/STI status and that of his or her partner. Still, the Centers for Disease Control and Prevention (CDC) estimates nearly 20 million new cases of these reportable STDs/STIs (gonorrhea, chlamydia, syphilis) occur each year in the United States—almost half of them among young people 15 to 24 years of age.
While not the most common STD/STI, HIV/AIDS is one of the most devastating and most well known. Recent data from the CDC indicate that 1.1 million Americans have HIV:
- One in five is unaware that they have the virus.
- Approximately 50,000 Americans become infected with HIV each year.
- 15,529 people with AIDS died in 2010.
More information about the number of people who test positive for HIV/AIDS in the United States and the differences in these numbers for individuals from different cultures and backgrounds is available at the CDC website.
What are the symptoms of a sexually transmitted disease or sexually transmitted infection (STD/STI)?
People with STDs/STIs may feel ill and notice some of the following signs and symptoms:
- Unusual discharge from the penis or vagina
- Sores or warts on the genital area
- Painful or frequent urination
- Itching and redness in the genital area
- Blisters or sores in or around the mouth
- Abnormal vaginal odor
- Anal itching, soreness, or bleeding
- Abdominal pain
- Fever
In some cases, people with STDs/STIs have no symptoms, and over time the symptoms, if present, can improve on their own. However, it is common for individuals to have an STD/STI and pass it on to others without knowing it.
If you are concerned that you or your sexual partner may have an STD/STI, talk to your health care provider. Even if you do not have symptoms, it is possible you may have an STD/STI that needs to be treated to ensure your and your partner's sexual health.
What causes a sexually transmitted disease or sexually transmitted infection (STD/STI)?
There are two major causes of STDs/STIs:
- Bacteria, including chlamydia, gonorrhea, and syphilis
- Viruses, including HIV/AIDS, herpes simplex virus, human papillomavirus (HPV), hepatitis B virus, and cytomegalovirus (CMV; pronounced sahy-toh-MEG-uh-loh-vahy-ruhs), yeasts and protozoan parasites, such as Trichomonas vaginalis (pronounced TRIK-uh-MOH-nuss vaj-uh-NAHY-lis), or insects such as crab lice or scabies mites, cause STDs/STIs.
Any STD/STI can be spread through sexual intercourse, and some STDs/STIs also are spread through oral sex and sex play. Ejaculation does not have to occur for an STD/STI to be passed from person to person. Sharing contaminated needles used to inject drugs or using contaminated body piercing and tattooing equipment also can transmit some infections, such as HIV or hepatitis B and C.
A few diseases (such as CMV and molluscum contagiosum) can be sexually transmitted but are also spread through nonsexual, close contact. Regardless of how a person is exposed, once a person is infected by an STD/STI, he or she can spread the infection to other people through oral, vaginal, or anal sex, even if he or she has no symptoms.
STDs/STIs are of particular concern in pregnant women, because some infections can be passed on to the infant before birth or during delivery. However, the risk of transmission from mother to infant can be lowered, and it is important for every expectant mother to be screened.
For example, HIV can be passed from mother to infant during pregnancy before birth, at the time of delivery, or after birth during breastfeeding. This transmission can be prevented through treatment with certain medications during pregnancy and near delivery. After birth, women who have HIV should refrain from breastfeeding their infants if safe alternatives, such as infant formula, are available, further reducing the infant's risk.
In other cases, if the mother has an infection such as gonorrhea or herpes, in which risks of transmission are high during delivery, other steps can be taken to reduce the likelihood that the infant will be infected. In these instances, health care providers can treat the pregnant woman for the STD/STI before birth, or the infant can be delivered by cesarean section (also referred to as C section).
CMV affects about 1% of all births. A pregnant woman infected with CMV can transmit the infection to the fetus in the womb, or it can be passed to the infant during delivery or by breastfeeding. She could also pass it to her newborn after birth if the child comes into contact with her body fluids (for example, saliva or urine) carrying the virus. If a health care provider suspects that a woman has a CMV infection during pregnancy, an ultrasound examination, blood tests, and other tests are done to assess the health of the fetus. Most infants who were infected with the virus during pregnancy do not have any detectable problems after birth. But, 10% or 20% will have serious problems, including deafness and intellectual disabilities. If fetal testing shows increased risk of serious problems, some women opt to end the pregnancy. Researchers are studying antiviral drugs, immune treatments, and other medical approaches to control the infection during pregnancy. Some research is focusing on vaccines to prevent CMV infection.
What are some types of sexually transmitted diseases or sexually transmitted infections (STDs/STIs)?
Approximately 20 different infections are known to be transmitted through sexual contact. Here are descriptions of some of the most common and well known:
- Chlamydia
- Gonorrhea
- Genital Herpes
- HIV/AIDS
- Human Papillomavirus (HPV)
- Syphilis
- Bacterial Vaginosis
- Trichomoniasis
- Viral Hepatitis
Chlamydia
Chlamydia1 (pronounced kla-MID-ee-uh) is a common STD/STI caused by the bacterium Chlamydia trachomatis. Chlamydia can be transmitted during vaginal, oral, or anal sexual contact with an infected partner. While many individuals will not experience symptoms, chlamydia can cause fever, abdominal pain, and unusual discharge of the penis or vagina.
In women, whether or not they are having symptoms and know about their infection, chlamydia can cause pelvic inflammatory disease (PID). In PID, the untreated STD/STI progresses and involves other parts of the woman's reproductive system, including the uterus and fallopian tubes. This progression can lead to permanent damage to the woman's reproductive organs. This damage may lead to ectopic pregnancy (in which the fetus develops in abnormal places outside of the womb, a condition that can be life-threatening) and infertility.
Additionally, if the woman is pregnant, her developing fetus is at risk, because chlamydia can be passed on during her pregnancy or delivery and could lead to eye infections or pneumonia in the infant. If chlamydia is detected early, it can be treated easily with an antibiotic taken by mouth.
Gonorrhea
Gonorrhea (pronounced gon-uh-REE-uh) is caused by the bacterium Neisseria gonorrhoeae, which can grow rapidly and multiply easily in the warm, moist areas of the reproductive tract. The most common symptoms of gonorrheal infection are a discharge from the vagina or penis and painful or difficult urination.
As with chlamydial infection, the most common and serious complications of gonorrhea occur in women and include pelvic inflammatory disease ((PID), ectopic pregnancy, infertility, and the potential spread to the developing fetus if acquired during pregnancy. Gonorrhea also can infect the mouth, throat, eyes, and rectum and can spread to the blood and joints, where it can become a life-threatening illness.
In addition, people with gonorrhea can more easily contract HIV, the virus that causes AIDS. HIV-infected people with gonorrhea are also more likely to transmit the virus to someone else.
Genital Herpes
Genital herpes is a contagious infection caused by the herpes simplex virus (HSV). There are two different strains, or types, of HSV: herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). Both can cause genital herpes, although most cases of genital herpes are caused by HSV-2.5 When symptomatic, HSV-1 usually appears as fever blisters or cold sores on the lips, but it can also infect the genital region through oral-genital or genital-genital contact. Symptomatic HSV-2 typically causes painful, watery skin blisters on or around the genitals or anus. However, substantial numbers of people who carry these viruses have no or only minimal signs or symptoms.
Neither HSV-1 nor HSV-2 can be cured, and even during times when an infected person has no symptoms, the virus can be found in the body's nerve cells. Periodically, some people will experience outbreaks in which new blisters form on the skin in the genital area; at those times, the virus is more likely to be passed on to other people.
Pregnant women, especially those who acquire genital herpes for the first time during pregnancy, may pass the infection to their newborns, causing life-threatening neonatal HSV, an infection affecting the infant's skin, brain, and other organs.
HIV/AIDS
HIV, or the human immunodeficiency virus, is the virus that causes AIDS (acquired immunodeficiency syndrome). HIV destroys the body's immune system by killing the blood cells that fight infection. Once HIV destroys a substantial proportion of these cells, the body's ability to fight off and recover from infections is compromised. This advanced stage of HIV infection is known as AIDS.
People whose HIV has progressed to AIDS are very susceptible to opportunistic infections that do not normally make people sick and to certain forms of cancer.
AIDS can be prevented by early initiation of antiretroviral therapy in those with HIV infection. Transmission of the virus primarily occurs during unprotected sexual activity and by sharing needles used to inject intravenous drugs, although the virus also can spread from mother to infant during pregnancy, delivery, and breastfeeding.
In 2013, NIH-supported researchers reported that a 2-year-old child who was born with HIV and was treated starting in the first few days of life has had her HIV infection go into remission. This appears to be the first case of functional cure of HIV.
Human Papillomavirus (HPV)
HPV is the most common STD/STI. More than 40 HPV types exist, and all of them can infect both men and women. The types of HPVs vary in their ability to cause genital warts; infect other regions of the body, including the mouth and throat; and cause cancers of the cervix, vulva, penis, and mouth.
Although no cure exists for HPV infection once it occurs, regular screening with a Pap smear test can prevent or detect at an early stage most cases of HPV-caused cervical cancer. (A Pap smear test involves a health care provider taking samples of cells from the cervix during a standard gynecologic exam; these cells are examined under a microscope for signs of developing cancer).
A newly available vaccine protects against most (but not all) HPV types that cause cervical cancer. The American Academy of Pediatrics recommends this vaccine for school-aged boys and girls.
Syphilis
Syphilis infections, caused by the bacterium Treponema pallidum, are passed from person to person during vaginal, anal, or oral sex through direct contact with sores, called chancres. Between 2001 and 2009, the Centers for Disease Control and Prevention (CDC) data show that the syphilis rate increased each year. Those people at highest risk for syphilis include men having sex with both men and women and people residing in the south. The first sign of syphilis is a chancre, a painless genital sore that most often appears on the penis or in and around the vagina. Beyond being the first sign of a syphilis infection, chancres make a person two to five times more likely to contract an HIV infection. If the person is already infected with HIV, chancres also increase the likelihood that the virus will be passed on to a sexual partner. These sores typically resolve on their own, even without treatment. However, the body does not clear the infection on its own, and, over time, syphilis may involve other organs, including the skin, heart, blood vessels, liver, bones, and joints in secondary syphilis. If the illness is still not treated, tertiary syphilis can develop over a period of years and involve the nerves, eyes, and brain and can potentially cause death.
Expectant mothers harboring the bacterium are at an increased risk of miscarriage and stillbirth, and they can pass the infection on to their fetuses during pregnancy and delivery. Infants that acquire congenital syphilis during pregnancy may suffer from skeletal deformity, difficulty with speech and motor development, seizure, anemia, liver disease, and neurologic problems.
Bacterial Vaginosis
Bacterial vaginosis is a common, possibly sexually transmitted, vaginal infection in women of reproductive age. While it is healthy and normal for a vagina to have bacteria, just like the skin, mouth, or gastrointestinal (GI) tract, sometimes changes in the balance of different types of bacteria can cause problems.
Bacterial vaginosis occurs when problematic bacteria that are normally present only in small amounts increase in number, replace normal vaginal lactobacilli bacteria, and upset the usual balance. This situation becomes more likely if a woman douches frequently or has new or multiple sexual partners. The most common sign of a bacterial vaginosis infection is a thin, milky discharge that is often described as having a "fishy" odor. However, some women will have no symptoms at all.
Regardless of symptoms, having bacterial vaginosis increases the risk of getting other STDs/STIs and is also associated with pelvic inflammatory disease (PID), an infection of the female reproductive organs, including the uterus and the fallopian tubes (which carry eggs to the uterus), and postoperative infections. Preterm labor and birth are also possibly more common in women with bacterial vaginosis.
Trichomoniasis
Trichomoniasis12 (pronounced trik-uh-muh-NAHY-uh-sis) infection is caused by the single-celled protozoan parasite Trichomonas vaginalis and is common in young, sexually active women. The parasite also infects men, though less frequently. The parasite can be transmitted between men and women as well as between women whenever physical contact occurs between the genital areas. Although Trichomonas infections do not always cause symptoms, they can cause frequent, painful, or burning urination in men and women as well as vaginal discharge, genital soreness, redness, or itching in women. Because the infection can occur without symptoms, a person may be unaware that he or she is infected and continue to re-infect a sexual partner who is having recurrent signs of infection. As with bacterial STDs/STIs, all sexual partners should be treated at the same time to avoid re-infection.
NICHD-sponsored research has shown that during pregnancy, Trichomonas infection is associated with an increased risk of premature birth and infants with low birth weight. Moreover, infants born to mothers with Trichomonas infection are more than twice as likely as infants born to uninfected women to be stillborn or to die as newborns.
Viral Hepatitis
Viral hepatitis is a serious liver disease that can be caused by several different viruses, which can be transmitted through sexual contact.
- Hepatitis A virus (HAV) causes a short-term or self-limited liver infection that can be quite serious, although it does not result in chronic infection. While there are other ways the virus can be transmitted, HAV can be spread from person to person during sexual activity through oral-rectal contact. Vaccination can prevent HAV infection.
- Hepatitis B virus (HBV) causes a serious liver disease that can result in both immediate illness and lifelong infection leading to permanent liver scarring (cirrhosis), cancer, liver failure, and death. HBV spreads through both heterosexual and homosexual contact as well as through contact with other bodily fluids, such as blood, through shared contaminated needles used for injecting intravenous (IV) drugs, tattooing, and piercing. Pregnant women with HBV can transmit the virus to their infants during delivery. HBV infection is preventable through vaccination.
- Hepatitis C virus (HCV) can cause an immediate illness affecting the liver, but it more commonly becomes a silent, chronic infection that leads to liver scarring (cirrhosis), cancer, liver failure, and death. HCV is most commonly transmitted through sharing needles or exposure to infected blood. However, it can spread through sexual contact or from mother to fetus during pregnancy and delivery. There is no vaccine for HCV, and treatments are not always effective.
How do health care providers diagnose a sexually transmitted disease or sexually transmitted infection (STD/STI)?
Any person who is sexually active should discuss his or her risk factors for STDs/STIs with a health care provider and ask about getting tested. If you are sexually active, it is important to remember that you may have an STD/STI and not know it because many STDs/STIs do not cause symptoms. You should get tested and have regular checkups with a health care provider who can help assess and manage your risk, answer your questions, and diagnose and treat an STD/STI if needed.
Starting treatment quickly is important to prevent transmission of infections to other people and to minimize the long-term complications of STDs/STIs. Recent sexual partners should also be treated to prevent re-infection and further transmission.
Some STDs/STIs may be diagnosed during a physical exam or through microscopic examination of a sore or fluid swabbed from the vagina, penis, or anus. This fluid can also be cultured over a few days to see whether infectious bacteria or yeast can be detected. The effects of human papilloma virus (HPV), which causes genital warts and cervical cancer, can be detected in a woman when her health care provider performs a pap smear test and takes samples of cells from the cervix to be checked microscopically for abnormal changes.1 Blood tests are used to detect infections such as hepatitis A, B, and C or HIV/AIDS.
Because sexually transmitted diseases are passed from person to person and can have serious health consequences, the health department notifies people if they have been exposed to certain STDs/STIs. Not all STDs/STIs are reported, though. If you receive a notice, it is important to see a health care provider, be tested, and start treatment right away.
Screening is especially important for pregnant women, because many STDs/STIs can be passed on to the fetus during pregnancy or delivery. During an early prenatal visit, with the help of her health care provider, an expectant mother should be screened for these infections, including HIV and syphilis. Some of these STDs/STIs can be cured with drug treatment, but not all of them. However, even if the infection is not curable, a pregnant woman can usually take measures to protect her infant from infection.
Is there a cure for sexually transmitted diseases and sexually transmitted infections (STDs/STIs)?
Viruses such as HIV, genital herpes, human papillomavirus, hepatitis, and cytomegalovirus cause STDs/STIs that cannot be cured. People with an STD/STI caused by a virus will be infected for life and will always be at risk of infecting their sexual partners, although for many viruses treatment significantly reduces this risk. Treatments are available to cure STDs/STIs caused by bacteria, yeast, or parasites.
What are the treatments for sexually transmitted diseases and sexually transmitted infections (STDs/STIs)?
STDs/STIs caused by bacteria, yeast, or parasites can be treated with antibiotics. These antibiotics are most often given by mouth (orally). However, sometimes they are injected or applied directly to the affected area. Whatever the infection, and regardless of how quickly the symptoms resolve after beginning treatment, the infected person must take all of the medicine prescribed by the health care provider to ensure that the STD/STI is completely treated.
Although treatments, complications, and outcomes vary among viral STDs/STIs depending on the particular virus (HIV, genital herpes, human papillomavirus, hepatitis, or cytomegalovirus), health care providers can provide treatments to reduce the symptoms and the progression of most of these illnesses. For example, medications are available to limit the frequency and severity of genital herpes outbreaks while reducing the risk that the virus will be passed on to other people.
Individuals with HIV need to take special antiretroviral drugs that control the amount of virus they carry. These drugs, called highly active antiretroviral therapy, or HAART,1 can help people live longer, healthier lives. If a woman with HIV becomes pregnant, these medicines also can reduce the chance that her fetus or infant will get the infection.
Being tested and treated for STDs/STIs is especially important for pregnant women because some STDs/STIs may be passed on to their infants during pregnancy or delivery. Testing women for these STDs/STIs early in their pregnancy is important, so that steps can be taken to help ensure delivery of a healthy infant. The necessary treatment will depend on the type of STD/STI involved.
Treatments for Specific Types of Sexually Transmitted Diseases and Sexually Transmitted Infections (STDs/STIs)
- Gonorrhea and Chlamydia
- Genital Herpes
- Human Papillomavirus (HPV)
- Syphilis
- Bacterial Vaginosis
- Trichomoniasis
- Viral Hepatitis
- HIV/AIDS
- During Pregnancy
Gonorrhea and Chlamydia
Gonorrhea and chlamydia are bacterial STDs/STIs that can be treated with antibiotics given either orally or by injection. Because the infections often occur together, people who have one infection are typically treated for both by their health care provider. Recent sexual partners should be treated at the same time.
Genital Herpes
Genital herpes outbreaks can be treated with antiviral drugs. Although this medication can limit the length and severity of outbreaks, it does not cure the infection. In addition, daily suppressive therapy (daily use of antiviral medication) for herpes can reduce the likelihood of transmission to partners. A pregnant woman known to have the infection must take additional care because she can pass the infection to her infant during delivery. Women who first acquire genital HSV during pregnancy are at highest risk of transmission to their infants. If a pregnant woman has an outbreak when she goes into labor, she may need to have a cesarean section (C-section) to prevent the infant from getting the virus during birth.
Human Papillomavirus (HPV)
A person who has an HPV infection cannot be cured. However, many HPV infections can be prevented with vaccination. Furthermore, a health care provider can treat genital warts caused by the virus as well as monitor and control a woman's risk of cervical cancer through frequent screening with Pap smear tests.
Syphilis
If recognized during the early stages, usually within the first year of infection, syphilis can be treated with a singular intramuscular injection of antibiotic. A person being treated for syphilis must avoid sexual contact until the chancre sores caused by the bacteria are completely healed to avoid infecting other people.
If a person does not recognize the infection early, or does not seek treatment immediately, longer treatment with antibiotics may be required. If left untreated, the infection can progress even further and potentially cause death. Although antibiotics can prevent the infection from getting worse, they cannot reverse damage that has already occurred.
Bacterial Vaginosis
Bacterial vaginosis can be treated with antibiotics, typically metronidazole or clindamycin. Generally, male sexual partners of women with bacterial vaginosis do not need to be treated because treatment of partners has not been shown to reduce the risk of recurrence.
Treatment during pregnancy is recommended primarily for women at risk for preterm labor or having a low birthweight infant.
Trichomoniasis
Trichomoniasis can be treated with a single dose of an antibiotic, usually either metronidazole or tinidazole, taken by mouth. Often, Trichomonas infection recurs, so it is important to make sure that both you and your sexual partners are treated if you are diagnosed with this infection.
Viral Hepatitis
- Hepatitis A virus (HAV) infects the liver and may cause abdominal pain, nausea, and vomiting. Usually the infection gets better on its own without requiring treatment. In some cases, however, individuals may have lasting damage to their livers or may have such severe nausea and vomiting that they must be admitted to the hospital.
- Hepatitis B virus (HBV) can cause a lifelong infection but can be treated with antiviral medications. People with HBV infection will need to see a liver specialist with experience treating individuals with chronic liver disease. These individuals need to take special care not to pass on the virus to their sexual partners, and sexual partners should receive hepatitis B vaccine if not already immune.
- Hepatitis C virus can cause immediate illness affecting the liver or, more commonly, it can be a silent, chronic infection. As with hepatitis B, individuals with HCV may have a lifelong infection and will always be at risk of passing the virus on to their sexual partners. New treatments are available that can clear the infection in some individuals.
HIV/AIDS
There is no cure for HIV/AIDS. However, research into new treatments has improved outcomes for people living with the disease. A combination of antiretroviral drugs can be given in highly active antiretroviral therapy to control the virus, promote a healthy immune system, help people with the virus live longer lives, and reduce the risk of transmission.
During Pregnancy
Pregnant women who have certain types of STDs/STIs may pass them on to their infants during pregnancy or delivery. Therefore, it is important for women to be tested for such STDs/STIs as part of their early prenatal care to help ensure delivery of a healthy infant.
The specific treatment will depend on which STD/STI is involved.
Sexually Transmitted Diseases (STDs): Other FAQs
Basic information for topics, such as “What is it?” and “How many people are affected?” is available in the Condition Information section. In addition, Frequently Asked Questions (FAQs) that are specific to a certain topic are answered in this section.
Am I at risk for STDs/STIs?
STDs/STIs affect men and women of all races, backgrounds, sexual orientations, and economic levels. Anyone who is having or has had vaginal, anal, or oral sex has some degree of risk for an STD/STI. In fact, some STDs/STIs can be passed through sexual play that does not involve intercourse.
You can analyze your risk for STDs/STIs with the STD Wizard—a free interactive online tool based on the Centers for Disease Control and Prevention's (CDC's) STD Treatment Guidelines. The STD Wizard recommends tests and vaccines based on your responses concerning some of your personal characteristics and behaviors. You can use these recommendations to start a discussion with your health care provider about your STD/STI risk and the tests you may need. The STD Wizard is available in both English and Spanish.
How can I avoid getting a sexually transmitted disease or sexually transmitted infection (STD/STI)?
The most reliable ways to avoid STDs/STIs are to abstain from sexual contact or to be in a long-term monogamous relationship with a partner who has been tested and is uninfected. In addition, the following measures can also help you avoid STDs/STIs:
- Know your sexual partner's STD/STI and health history.
- Talk to your health care provider about your risk, and get tested for STDs/STIs.
- Get vaccinated against hepatitis A virus (HAV), hepatitis B virus (HBV), and human papillomavirus (HPV).
- Use latex condoms correctly and consistently.
Remember, however, that while condoms greatly reduce the chance of getting certain STDs/STIs, such as gonorrhea, condoms cannot fully protect against infection because viruses and some bacteria can be passed from person to person by skin-to-skin contact in the genital area not covered by a condom
What should I do if I have been diagnosed with an STD/STI?
You should see your health care provider for treatment as soon as possible after receiving a diagnosis of an STD/STI. You also should notify, either yourself or with the help of the local health department, all recent sex partners and advise them to see their health care providers and be treated. These steps will reduce your risk of becoming re-infected, help avoid spreading the STD/STI to other people, and decrease the risk that your previous sexual partners will develop serious complications from the STD/STI. You and all of your sex partners must avoid sex until treatment is complete and all symptoms have disappeared.
In the case of STDs/STIs caused by viruses with no cure (for example, HIV, genital herpes, or hepatitis), special care and preventive measures can help control the infection, limit symptoms, and help maximize health.
Are there disorders or conditions associated with sexually transmitted diseases and sexually transmitted infections (STDs/STIs)?
STDs/STIs in women can cause pelvic inflammatory disease (PID), which may result in infertility (difficulty getting pregnant).
Men with STDs/STIs also can have problems with infertility.
Additionally, a person with an STD/STI other than HIV is two to five times more likely to contract the HIV virus than a person without an STD/STI. If a person is already HIV positive, having another STD/STI increases the chances that he or she will pass the virus on to his or her sexual partner.
Some STDs/STIs, such as human papillomavirus, viral hepatitis, and HIV, increase the risk of some forms of cancer.
Certain STDs/STIs can pass from a pregnant woman to the fetus in her womb. The effects can be life threatening, as is the case with HIV. Other STDs/STIs can cause a range of disorders in the infant, including deafness, blindness, and intellectual disability.
Can a sexually transmitted disease or sexually transmitted infection (STD/STI) lead to cancer?
Having an STD/STI increases a person's risk for several types of cancer.
Certain high-risk types of human papillomavirus (HPV) can cause cervical cancer in women. In men, HPV infection can lead to the development of penile cancers. HPV also can cause cancers of the mouth, throat, and anus in both sexes.
Acquiring viral hepatitis B or C puts a person at risk for liver cancer, and untreated HIV/AIDS increases risk for several types of rare cancers, including lymphomas, sarcomas, and cervical cancer
If I have a sexually transmitted disease or sexually transmitted infection (STD/STI), will I be able to get pregnant?
Having an STD/STI will not prevent a woman from getting pregnant.
However, in some instances, women who have had STDs/STIs may have difficulty getting pregnant because of scarring and damage to their reproductive organs leading to infertility. This situation is particularly common in women who have had pelvic inflammatory disease. Additionally, early during pregnancy, STDs/STIs may increase the risk of miscarriage
What is the link between sexually transmitted diseases or sexually transmitted infections (STDs/STIs) and infertility?
In most cases, STDs/STIs are linked to infertility primarily when they are left untreated.
For instance, chlamydia and gonorrhea are sexually transmitted bacterial infections that can be cured easily with antibiotics. Left untreated, 10% to 20% of chlamydial and gonorrheal infections in women can result in pelvic inflammatory disease (PID)—a condition that can cause long-term complications, such as chronic pelvic pain, ectopic pregnancy (pregnancy outside of the uterus), and infertility.
Additionally, infections with gonorrhea and chlamydia may not cause symptoms and may go unnoticed. These undiagnosed and untreated infections can lead to severe health consequences, especially in women, causing permanent damage to reproductive organs.
The Ccenters for Disease Control and Prevention estimates that these infections cause infertility in at least 24,000 women each year. Although infertility is less common among men, it does occur. More commonly, untreated chlamydia and gonorrhea infections in men may cause epididymitis, a painful infection in the tissue surrounding the testicles, or urethritis, an infection of the urinary canal in the penis, which causes painful urination and fever.
Additional information on PID is available from the National Institute of Allergy and Infectious Diseases.
How do sexually transmitted diseases and sexually transmitted infections (STDs/STIs) affect pregnancy?
STDs/STIs pose special risks for pregnant women and their infants.
If a mother has an STD/STI, it is possible for the fetus or newborn to become infected. Some STDs/STIs, including chlamydia, gonorrhea, genital herpes, and cytomegalovirus can be passed from mother to infant during delivery when the infant passes through an infected birth canal. A few STDs/STIs, including syphilis, HIV, and CMV, can infect a fetus before birth during the pregnancy. It is important for a pregnant woman to be tested for STDs/STIs, including HIV/AIDS and syphilis, as a part of her prenatal care.
STD/STI testing as a part of prenatal care can determine if an expectant mother or her sexual partner has an infection that can be cured with drug treatment. Early treatment decreases the chances that the infant will contract the disease. While not all STDs/STIs can be cured, the mother and her health care provider can take steps to protect her and her infant.
To reduce the chance of certain STDs/STIs spreading to the infant during delivery, the health care provider might recommend a cesarean delivery.
In most hospitals, infants’ eyes are routinely treated with an antibiotic ointment shortly after birth. This is to prevent blindness due to exposure to gonorrhea or chlamydia bacteria during delivery if the pregnant woman had an undetected infection.
STDs/STIs during pregnancy can also cause:
- Miscarriage
- Ectopic pregnancy (when the embryo implants outside of the uterus, usually in a fallopian tube)
- Preterm labor and delivery (before 37 completed weeks of pregnancy)
- Low birth weight
- Birth defects, including blindness, deafness, bone deformities, and intellectual disability
- Stillbirth
- Illness in the newborn period (first month of life)
- Newborn death
STDs/STIs are of special concern during pregnancy and pose significant health risks to unborn infants:
- HIV/AIDS. HIV can be passed from mother to infant during pregnancy before birth, at the time of delivery, or after birth during breastfeeding.
- Gonorrhea. If a pregnant woman has a gonorrheal infection, she may give the infection to her infant as the infant passes through the birth canal during delivery. This infection in an infant can cause eye infections, pneumonia, or infections of the joints or blood. Treating gonorrhea as soon as it is detected in pregnant women will reduce the risk of transmission.
- Chlamydia. Similar to a gonorrheal infection, a chlamydial infection at the time of delivery can lead to eye infections or pneumonia in the infant. However, chlamydial infection during pregnancy also has been associated with an increased risk of preterm birth and its complications.
- Genital herpes. Pregnant women newly infected with genital herpes late in pregnancy have a 30% to 60% chance of infecting the infant they carry. The risk of infection is particularly high during delivery. Herpes infections in newborns are serious and potentially life-threatening. Infection with the herpes virus during pregnancy or at the time of delivery can lead to brain damage, blindness, and damage to other organs.
- If a pregnant woman has had genital herpes in the past, there are medications that she can take to reduce the chance that she will have an outbreak and thus reduce the risk to her infant.
- If a woman has active herpes sores when she goes into labor, the infant can be delivered by cesarean section to reduce the chance that the infant will come in contact with the virus.
- Hepatitis B virus. If a woman is infected with the hepatitis B virus during pregnancy, the virus also could infect her fetus. The likelihood of this occurrence depends on when the mother was infected. If the mother acquires the infection early in her pregnancy, the chance that the virus will infect her fetus is less than 10%. However, if the infection occurs later in her pregnancy, the risk goes up to 90%. Hepatitis B can be severe in infants and can threaten their lives. It also can lead to liver scarring, failure, and cancer, which can be fatal in up to 25% of cases. In addition, infected newborns have a very high risk of becoming carriers of the hepatitis B virus and can spread the infection to others.
- In some cases, if a woman is exposed to hepatitis B during pregnancy, she may be treated with a special antibody to reduce the likelihood that she will get the infection. All healthy infants should be vaccinated against hepatitis B to give them lifelong protection against the virus. Infants born to women with evidence of ongoing hepatitis B infection (hepatitis B surface antigen positive) should also receive hepatitis B hyperimmune globulin as soon as possible after birth.
- Cytomegalovirus (CMV). CMV is a common virus present in many body fluids that can be spread through close personal contact, such as kissing or sharing eating utensils, as well as sexual contact. The virus is common in the general population and usually does not cause health problems. However, if an expectant mother acquires the virus for the first time during pregnancy, the risk is high that she will pass it on to her infant. Unfortunately, a pregnant woman may not even know she has the infection, and she may still pass the virus on to her infant.7 CMV in an infant can lead to serious illness, lasting disabilities, or death. Each year in the United States, an estimated 40,000 infants are born with CMV infection, causing an estimated 400 deaths and leaving about 8,000 infants with permanent disabilities, such as hearing or vision loss, or intellectual disability. Currently routine screening for CMV in pregnancy is not recommended. Researchers are working on treatments for CMV and also vaccines to try to prevent new infections during pregnancy and to reduce the risk of transmission to the infant.
Sexually Transmitted Diseases (STDs): NICHD Research Information
STDs/STIs have enormous effects on the health of individuals and society. Consequently, STDs/STIs are an active focus of the NICHD’s research. While many effective, commonly available interventions can reduce the incidence of STDs/STIs, these interventions are not always implemented, in part because of complicating social factors. As a result, STDs/STIs are often difficult to prevent, diagnose, and treat, and this situation has a serious impact on public health. The NICHD’s portfolio covers a variety of topics in STDs/STI research with a focus on understanding epidemiology and improving screening, education, and preventative health interventions.
Sexually Transmitted Diseases (STDs): NICHD Research Goals
The NICHD focuses a great deal of its research on factors and behaviors that affect the risk of contracting or spreading STDs/STIs and works to develop new interventions to prevent the spread of STDs/STIs in vulnerable populations. Also, since 1987, the NICHD has supported research and training activities that have helped to establish and define the field of contraceptive microbicides, products that both prevent pregnancy and reduce the transmission of STDs, including HIV.
NICHD-supported researchers are also trying to understand the best ways to communicate with people about STDs/STIs and effective preventive measures. Some of this research includes studying attitudes, perception, and knowledge of STDs/STIs and their prevention; understanding the use and misuse of contraception to prevent STDs/STIs; and understanding the misinformation surrounding the topic while aiming to identify the best settings for providing education about sexual health.
Because preventing and treating STDs/STIs is a major goal for the NIH and its Institutes, the NICHD conducts and supports a variety of clinical trials on STDs/STIs, including HIV
NIAID: National Institute of Allergy and Infectious Diseases, NIH
back-to-top
Pelvic Inflammatory Disease (PID) - CDC Fact Sheet
Untreated sexually transmitted diseases (STDs) can cause pelvic inflammatory disease (PID), a serious condition, in women. 1 in 8 women with a history of PID experience difficulties getting pregnant. You can prevent PID if you know how to protect yourself.
What is PID?
Pelvic inflammatory disease is an infection of a woman’s reproductive organs. It is a complication often caused by some STDs, like chlamydia and gonorrhea. Other infections that are not sexually transmitted can also cause PID.
How do I get PID?
You are more likely to get PID if you
- Have an STD and do not get treated;
- Have more than one sex partner;
- Have a sex partner who has sex partners other than you;
- Have had PID before;
- Are sexually active and are age 25 or younger;
- Douche;
- Use an intrauterine device (IUD) for birth control.
How can I reduce my risk of getting PID?
The only way to avoid STDs is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting PID:
- Being in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Using latex condoms the right way every time you have sex.
How do I know if I have PID?
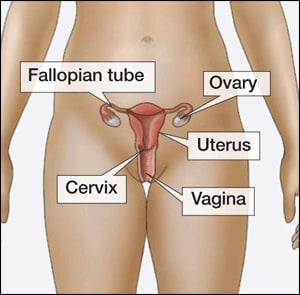
There are no tests for PID. A diagnosis is usually based on a combination of your medical history, physical exam, and other test results. You may not realize you have PID because your symptoms may be mild, or you may not experience any symptoms. However, if you do have symptoms, you may notice
- Pain in your lower abdomen;
- Fever;
- An unusual discharge with a bad odor from your vagina;
- Pain and/or bleeding when you have sex;
- Burning sensation when you urinate; or
- Bleeding between periods.
You should
- Be examined by your doctor if you notice any of these symptoms;
- Promptly see a doctor if you think you or your sex partner(s) have or were exposed to an STD;
- Promptly see a doctor if you have any genital symptoms such as an unusual sore, a smelly discharge, burning when peeing, or bleeding between periods;
- Get a test for chlamydia every year if you are sexually active and 25 years of age or younger;
- Have an honest and open talk with your health care provider if you are sexually active and ask whether you should be tested for other STDs.
Can PID be cured?
Yes, if PID is diagnosed early, it can be treated. However, treatment won’t undo any damage that has already happened to your reproductive system. The longer you wait to get treated, the more likely it is that you will have complications from PID. While taking antibiotics, your symptoms may go away before the infection is cured. Even if symptoms go away, you should finish taking all of your medicine. Be sure to tell your recent sex partner(s), so they can get tested and treated for STDs, too. It is also very important that you and your partner both finish your treatment before having any kind of sex so that you don’t re-infect each other.
You can get PID again if you get infected with an STD again. Also, if you have had PID before, you have a higher chance of getting it again.
What happens if I don't get treated?
If diagnosed and treated early, the complications of PID can be prevented. Some of the complications of PID are
- Formation of scar tissue both outside and inside the fallopian tubes that can lead to tubal blockage;
- Ectopic pregnancy (pregnancy outside the womb);
- Infertility (inability to get pregnant);
- Long-term pelvic/abdominal pain.
Pelvic Inflammatory Disease (PID) - CDC Fact Sheet
Detailed fact sheets are intended for physicians and individuals with specific questions about sexually transmitted diseases. Detailed fact sheets include specific testing and treatment recommendations as well as citations so the reader can research the topic more in depth.
What is pelvic inflammatory disease?
Pelvic inflammatory disease (PID) is a clinical syndrome that results from the ascension of microorganisms from the cervix and vagina to the upper genital tract. PID can lead to infertility and permanent damage of a woman’s reproductive organs.
How do women get pelvic inflammatory disease?
Women develop PID when certain bacteria, such as chlamydia or gonorrhea, move upward from a woman's vagina or cervix into her reproductive organs. PID is a serious complication of some sexually transmitted diseases (STDs), especially chlamydia and gonorrhea.
What causes pelvic inflammatory disease?
Many different types of microorganisms can cause PID; therefore, it is a considered a polymicrobial infection. Most cases of PID are caused by gonorrhea and chlamydia. Sexually transmitted disease pathogens Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) have frequently been identified among women with PID infection and these microbes have accounted for a third to a half of the cases While the focus has been on the role played by STDs, especially CT in the etiology of PID, endogenous microorganisms, including gram positive and negative anaerobic organisms and aerobic/facultative gram positive and negative rods and cocci, found at high levels in women with bacterial vaginosis, also have been implicated in the pathogenesis of PID. Newer data suggest that Mycoplasm genitalium may also play a role in PID and may be associated with milder symptoms Because of this polymicrobial nature, broad-spectrum regimens that provide adequate coverage of likely pathogens are recommended.
What are the signs and symptoms of pelvic inflammatory disease?
Since some women with PID have subtle or mild symptoms, many episodes of PID can go unrecognized by women and their health care providers. Women with PID present with a variety of clinical signs and symptoms that range from subtle and mild to severe. Some of the signs and symptoms associated with acute PID are nonspecific, so it is important to consider other reproductive tract illnesses and diseases of both the urinary and the gastrointestinal tracts during an evaluation of a sexually active female with lower abdominal pain. Pregnancy must also be excluded. A pregnancy test should always be performed to exclude ectopic pregnancy and because PID can occur concurrently with pregnancy. In the case of subclinical PID, women have mild or no pelvic pain, despite evidence of endometritis or salpingitis.
When symptoms are present, the most common symptoms of PID are
- Lower abdominal pain
- Mild pelvic pain
- Increased vaginal discharge
- Irregular menstrual bleeding
- Fever (>38° C)
- Pain with intercourse
- Painful and frequent urination
- Abdominal tenderness
- Pelvic organ tenderness
- Uterine tenderness (along with endometriosis)
- Adnexal tenderness (along with salpingitis)
- Cervical motion tenderness
- Inflammation
What are the complications of pelvic inflammatory disease?
The risk of developing short and long-term complications from PID depends upon the severity and number of episodes of PID, thereby emphasizing the importance of prompt and appropriate treatment.
Complications of PID include
- Scarred fallopian tube
- Tubo-ovarian abscess (TOA)
- Tubal factor infertility
- Ectopic pregnancy
- Pelvic adhesions
- Chronic pelvic pain
Complications of PID such as chronic pelvic pain and scarring are difficult to treat but sometimes improve with surgery.
Tubo-ovarian abscess (TOA) is a serious short-term complication of PID that is characterized by an inflammatory mass involving the fallopian tube, ovary, and, occasionally, other adjacent pelvic organs. The microbiology of TOAs is similar to PID and the diagnosis necessitates initial hospital admission. Treatment includes broad-spectrum antibiotics with or without a drainage procedure, with surgery often reserved for patients with suspected rupture or who fail to respond to antibiotics. Women infected with HIV may be at higher risk for TOA. Mortality from PID is less than 1% and is usually secondary to rupture of a TOA or to ectopic pregnancy.
How is pelvic inflammatory disease diagnosed?
There are no tests specifically for diagnosing PID. Because the diagnosis of PID can be imprecise, clinicians should have a high index of suspicion for the diagnosis. Physical examination findings to detect PID can also vary, and there is no single finding that is sensitive and specific for the diagnosis. When the diagnosis of PID is questionable, or when the illness is severe or not responding to therapy, further investigation may be warranted using other invasive procedures (endometrial biopsy, transvaginal ultrasonography or magnetic resonance imaging, and/or laparoscopy).
What medical examinations and assessments can be performed to diagnose pelvic inflammatory disease?
Clinical providers should perform a physical examination assessing the abdomen for tenderness. Other assessments may include evaluation of the vaginal pH, a whiff test (performed by adding a small amount of potassium hydroxide to a microscopic slide containing the vaginal discharge), and microscopy of the vaginal secretions for the presence of leukocytes, clue cells, and trichomonads.
The cervix should be examined for abnormal cervical and vaginal discharge, and friability. An internal exam should be performed to assess for pelvic organ tenderness and/or a mass. A clinician should also check for fever and for laboratory documentation of cervical infection with NG and CT. However, laboratory confirmation is not necessary to justify initiation of therapy for PID.
A serologic test for human immunodeficiency virus (HIV) is also recommended. A pregnancy test should always be performed to exclude ectopic pregnancy and because PID can occur concurrently with pregnancy. When the diagnosis of PID is questionable, or when the illness is severe or not responding to therapy, further investigation may be warranted using other invasive procedures (endometrial biopsy, transvaginal ultrasonography, magnetic resonance imaging, or laparoscopy).
A bimanual pelvic examination can be performed and may reveal pelvic organ tenderness, uterine tenderness in the case of endometritis, and adnexal tenderness in the case of salpingitis.
Cervical motion tenderness is another common finding in women with PID. Assessment of the lower genital tract (mucopurulent cervical discharge or evidence of WBCs on a microscopic evaluation of a saline preparation of vaginal fluid) can also yield signs of inflammation, which are consistent with the diagnosis of PID.
How is pelvic inflammatory disease treated?
Several types of antibiotics can cure PID. Antibiotic treatment does not, however, reverse any scarring caused by the infection. For this reason, it is critical that a woman receive care immediately if she has pelvic pain or other symptoms of PID. Prompt antibiotic treatment can prevent severe damage to the reproductive organs. The longer a woman delays treatment for PID, the more likely she is to become infertile or to have a future ectopic pregnancy because of damage to the fallopian tubes.
PID is usually treated with antibiotics to provide empiric, broad spectrum coverage of likely pathogens. Recommended regimens can be found in the 2010 STD Treatment Guidelines. Health care providers should emphasize to their patients that although their symptoms may go away before the infection is cured, they should finish taking all of the prescribed medicine. Additionally, a woman’s sex partner(s) should be treated to decrease the risk of re-infection, even if the partner(s) has no symptoms. Although sex partners may have no symptoms, they may still be infected with the organisms that can cause PID.
In certain cases, clinicians may recommend hospitalization to treat PID. This decision should be based on the judgment of the health care provider and the use of suggested criteria found in the 2010 STD Treatment Guidelines. If a woman’s symptoms continue, or if an abscess does not resolve, surgery may be needed.
What should a patient do after being diagnosed with pelvic inflammatory disease?
A patient should abstain from sexual intercourse until she and her partner(s) have completed treatment. Female latex condoms are also an option if a woman prefers them or if her male partner chooses not to use male condoms. Women who are told they have an STD and are treated for it should notify all of their recent sex partners so they can see a health care provider and be evaluated for STDs.
The diagnosis of PID provides an opportunity to educate adolescent and young women about prevention of STDs, including abstinence, consistent use of barrier methods of protection, immunization, and the importance of receiving periodic screening for STDs and HIV.
How can pelvic inflammatory disease be prevented?
Latex condoms may reduce the risk of PID. Treating STDs early can prevent PID. Since STDs play a major role in PID, screening and early treatment of infected women and their sex partners can help to minimize the risk of acquisition and continued transmission of STDs and subsequent adverse sequelae. Identifying, testing, and treating women at increased risk of cervical chlamydial infection can reduce the incidence of PID.
Consistent and correct use of latex male condoms can reduce the risk of transmission of chlamydia and gonorrhea and the risk of PID.
CDC recommends that providers screen the following populations for chlamydia and gonorrhea: all sexually active women younger than 25 years, as well as older women with risk factors such as new or multiple sex partners, or a sex partner who has a sexually transmitted infection.
What are the risk factors for developing pelvic inflammatory disease?
Risk factors are important considerations in both the clinical management and prevention of upper genital tract infections. As STDs are the most common etiological agents for PID, risk factors for developing PID can be related to those associated with the acquisition of STDs, including early coitarche, young age, alcohol use, inconsistent use of barrier contraceptives, and multiple sexual partners or having one partner with multiple sexual partners. Other factors that have been associated with PID include a prior history of PID, intrauterine device use (IUD) (risk seems to be primarily restricted to the first several weeks following insertion), and douching.
Is the number of women in the United States being diagnosed with pelvic inflammatory disease increasing?
No. Over the last decade, there have been several studies published suggesting overall declines in PID diagnosis in both hospital and ambulatory settings. While no single explanation exists for this declining trend, some have suggested that changes in sexually transmitted disease (STD) rates, increases in chlamydia screening coverage, availability of antimicrobial therapies that increase adherence to treatment, and more sensitive diagnostic technologies, could be impacting PID rates.
Despite declining trends, PID is a frequent and important infection that occurs among women of reproductive age. Based on a nationally representative sample from 2006-2010, 5.0% of U.S. women have reported being treated for PID in their lifetime.
The significant burden of disease attributed to PID comes predominantly from the long-term reproductive sequelae of tubal infection: tubal factor infertility, ectopic pregnancy, and pelvic adhesions, which can lead to chronic pelvic pain. Our knowledge of the longitudinal outcomes for affected women who experience PID is primarily derived from data published using a Scandinavian cohort of inpatients diagnosed with PID. Data from this study indicated that those women with PID were more likely to have ectopic pregnancy (6 times increased rate), tubal factor infertility (ranging 8% after first episode to as high as 40% after three episodes) and chronic pelvic pain (18% following 1 episode).
What is the economic burden of pelvic inflammatory disease in the United States?
A decline in incidence of PID is also reflected in the most recent cost estimates of PID and its sequelae. Direct medical expenditures for PID and its sequelae were estimated at $1.88 billion in 1998, compared to approximately $2.7 billion estimated in 1990. Based on a nationally representative sample from 2006-2010, approximately 4.2% of U.S. women have reported being treated for PID in their lifetime.
How can clinicians manage PID?
A critical component to the outpatient management is short-term follow-up, especially in the adolescent population. Since many adolescent women rely on outpatient services for the evaluation and treatment of STD symptoms, the need for a low diagnostic and management threshold for PID is even more critical, as the likelihood for additional follow-up care is low.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
www.cdc.gov/std
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
Sources
American College of Obstetricians and Gynecologists (ACOG). Pelvic Inflammatory Disease. ACOG Patient Education Pamphlet, 1999.
Westrom L and Eschenbach D. In: K. Holmes, P. Sparling, P. Mardh et al (eds). Sexually Transmitted Diseases, 3rd Edition. New York: McGraw-Hill, 1999, 783-809.
Source: CDC, HHS
back-to-top
Bacterial Vaginosis – CDC Fact Sheet

Any woman can get bacterial vaginosis. Having bacterial vaginosis can increase your chance of getting an STD.
What is bacterial vaginosis?
Bacterial vaginosis (BV) is an infection caused when too much of certain bacteria change the normal balance of bacteria in the vagina.
How common is bacterial vaginosis?
Bacterial vaginosis is the most common vaginal infection in women ages 15-44.
How is bacterial vaginosis spread?
We do not know about the cause of BV or how some women get it. BV is linked to an imbalance of “good” and “harmful” bacteria that are normally found in a woman's vagina.
We do know that having a new sex partner or multiple sex partners and douching can upset the balance of bacteria in the vagina and put women at increased risk for getting BV.
However, we do not know how sex contributes to BV. BV is not considered an STD, but having BV can increase your chances of getting an STD. BV may also affect women who have never had sex.
You cannot get BV from toilet seats, bedding, or swimming pools.
How can I avoid getting bacterial vaginosis?
Doctors and scientists do not completely understand how BV is spread, and there are no known best ways to prevent it.
The following basic prevention steps may help lower your risk of developing BV:
- Not having sex;
- Limiting your number of sex partners; and
- Not douching.
STDs & Pregnancy
I’m pregnant. How does bacterial vaginosis affect my baby?
Pregnant women can get BV. Pregnant women with BV are more likely to have babies who are born premature (early) or with low birth weight than women who do not have BV while pregnant. Low birth weight means having a baby that weighs less than 5.5 pounds at birth.
Treatment is especially important for pregnant women.
How do I know if I have bacterial vaginosis?
Many women with BV do not have symptoms. If you do have symptoms, you may notice a thin white or gray vaginal discharge, odor, pain, itching, or burning in the vagina. Some women have a strong fish-like odor, especially after sex. You may also have burning when urinating; itching around the outside of the vagina, or both.
How will my doctor know if I have bacterial vaginosis?
A health care provider will look at your vagina for signs of BV and perform laboratory tests on a sample of vaginal fluid to determine if BV is present.
Can bacterial vaginosis be cured?
BV will sometimes go away without treatment. But if you have symptoms of BV you should be checked and treated. It is important that you take all of the medicine prescribed to you, even if your symptoms go away. A health care provider can treat BV with antibiotics, but BV can recur even after treatment. Treatment may also reduce the risk for STDs.
Male sex partners of women diagnosed with BV generally do not need to be treated. However, BV may be transferred between female sex partners.
What happens if I don't get treated?
BV can cause some serious health risks, including
- Increasing your chance of getting HIV if you have sex with someone who is infected with HIV;
- If you are HIV positive, increasing your chance of passing HIV to your sex partner;
- Making it more likely that you will deliver your baby too early if you have BV while pregnant;
- Increasing your chance of getting other STDs, such as chlamydia and gonorrhea. These bacteria can sometimes cause pelvic inflammatory disease (PID), which can make it difficult or impossible for you to have children.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
www.cdc.gov/std
Order Publication Online at www.cdc.gov/std/pub
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
CDC National Prevention Information Network (NPIN)
P.O. Box 6003
Rockville, MD 20849-6003
E-mail: npin-info@cdc.gov
American Sexual Health Association (ASHA)
P. O. Box 13827
Research Triangle Park, NC 27709-3827
1-800-783-9877
Sources
Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. MMWR 2010;59(No. RR-12)
Hillier S and Holmes K. Bacterial vaginosis. In: K. Holmes, P. Sparling, P. Mardh et al (eds). Sexually Transmitted Diseases, 3rd Edition. New York: McGraw-Hill, 1999, 563-586.
CDC: Centers for Disease Control and Prevention
back-to-top
Chlamydia - CDC Fact Sheet
Chlamydia is a common sexually transmitted disease (STD) that can be easily cured. If left untreated, chlamydia can make it difficult for a woman to get pregnant.
What is chlamydia?
Chlamydia is a common STD that can infect both men and women. It can cause serious, permanent damage to a woman's reproductive system, making it difficult or impossible for her to get pregnant later on. Chlamydia can also cause a potentially fatal ectopic pregnancy (pregnancy that occurs outside the womb).
How is chlamydia spread?
You can get chlamydia by having vaginal, anal, or oral sex with someone who has chlamydia.
If your sex partner is male you can still get chlamydia even if he does not ejaculate (cum).
If you’ve had chlamydia and were treated in the past, you can still get infected again if you have unprotected sex with someone who has chlamydia.
If you are pregnant, you can give chlamydia to your baby during childbirth.
How can I reduce my risk of getting chlamydia?
The only way to avoid STDs is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting chlamydia:
- Being in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Using latex condoms the right way every time you have sex.
Am I at risk for chlamydia?
Anyone who has sex can get chlamydia through unprotected vaginal, anal, or oral sex. However, sexually active young people are at a higher risk of getting chlamydia. This is due to behaviors and biological factors common among young people. Gay, bisexual, and other men who have sex with men are also at risk since chlamydia can be spread through oral and anal sex.
Have an honest and open talk with your health care provider and ask whether you should be tested for chlamydia or other STDs. If you are a sexually active woman younger than 25 years, or an older woman with risk factors such as new or multiple sex partners, or a sex partner who has a sexually transmitted infection, you should get a test for chlamydia every year. Gay, bisexual, and men who have sex with men; as well as pregnant women should also be tested for chlamydia.
STDs & Pregnancy
I'm pregnant. How does chlamydia affect my baby?
If you are pregnant and have chlamydia, you can pass the infection to your baby during delivery. This could cause an eye infection or pneumonia in your newborn. Having chlamydia may also make it more likely to deliver your baby too early.
If you are pregnant, you should be tested for chlamydia at your first prenatal visit. Testing and treatment are the best ways to prevent health problems.
How do I know if I have chlamydia?
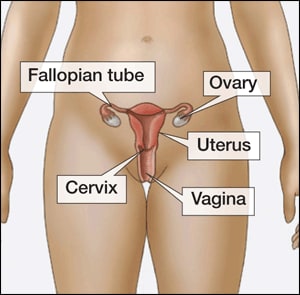
Most people who have chlamydia have no symptoms. If you do have symptoms, they may not appear until several weeks after you have sex with an infected partner. Even when chlamydia causes no symptoms, it can damage your reproductive system.
Women with symptoms may notice
- An abnormal vaginal discharge;
- A burning sensation when urinating.
Symptoms in men can include
- A discharge from their penis;
- A burning sensation when urinating;
- Pain and swelling in one or both testicles (although this is less common).
Men and women can also get infected with chlamydia in their rectum, either by having receptive anal sex, or by spread from another infected site (such as the vagina). While these infections often cause no symptoms, they can cause
- Rectal pain;
- Discharge;
- Bleeding.
You should be examined by your doctor if you notice any of these symptoms or if your partner has an STD or symptoms of an STD, such as an unusual sore, a smelly discharge, burning when urinating, or bleeding between periods.
How will my doctor know if I have chlamydia?
There are laboratory tests to diagnose chlamydia. Your health care provider may ask you to provide a urine sample or may use (or ask you to use) a cotton swab to get a sample from your vagina to test for chlamydia.
Can chlamydia be cured?
Yes, chlamydia can be cured with the right treatment. It is important that you take all of the medication your doctor prescribes to cure your infection. When taken properly it will stop the infection and could decrease your chances of having complications later on. Medication for chlamydia should not be shared with anyone.
Repeat infection with chlamydia is common. You should be tested again about three months after you are treated, even if your sex partner(s) was treated.
I was treated for chlamydia. When can I have sex again?
You should not have sex again until you and your sex partner(s) have completed treatment. If your doctor prescribes a single dose of medication, you should wait seven days after taking the medicine before having sex. If your doctor prescribes a medicine for you to take for seven days, you should wait until you have taken all of the doses before having sex.
STDs & Infertility
What happens if I don't get treated?
The initial damage that chlamydia causes often goes unnoticed. However, chlamydia can lead to serious health problems.
If you are a woman, untreated chlamydia can spread to your uterus and fallopian tubes (tubes that carry fertilized eggs from the ovaries to the uterus), causing pelvic inflammatory disease (PID). PID often has no symptoms, however some women may have abdominal and pelvic pain. Even if it doesn’t cause symptoms initially, PID can cause permanent damage to your reproductive system and lead to long-term pelvic pain, inability to get pregnant, and potentially deadly ectopic pregnancy (pregnancy outside the uterus).
Men rarely have health problems linked to chlamydia. Infection sometimes spreads to the tube that carries sperm from the testicles, causing pain and fever. Rarely, chlamydia can prevent a man from being able to have children.
Untreated chlamydia may also increase your chances of getting or giving HIV – the virus that causes AIDS.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
www.cdc.gov/std
American Sexual Health Association (ASHA)
P.O. Box 13827
Research Triangle Park, NC 27709-3827
1-800-783-9877
CDC:Centers for Disease Control and Prevention
back-to-top
Gonorrhea - CDC Fact Sheet

Anyone who is sexually active can get gonorrhea. Gonorrhea can cause very serious complications when not treated, but can be cured with the right medication.
What is gonorrhea?
Gonorrhea is a sexually transmitted disease (STD) that can infect both men and women. It can cause infections in the genitals, rectum, and throat. It is a very common infection, especially among young people ages 15-24 years.
How is gonorrhea spread?
You can get gonorrhea by having vaginal, anal, or oral sex with someone who has gonorrhea. A pregnant woman with gonorrhea can give the infection to her baby during childbirth.
How can I reduce my risk of getting gonorrhea?
The only way to avoid STDs is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting gonorrhea:
- Being in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Using latex condoms the right way every time you have sex.
Am I at risk for gonorrhea?
Any sexually active person can get gonorrhea through unprotected vaginal, anal, or oral sex.
If you are sexually active, have an honest and open talk with your health care provider and ask whether you should be tested for gonorrhea or other STDs. If you are a sexually active man who is gay, bisexual, or who has sex with men, you should be tested for gonorrhea every year. If you are a sexually active women younger than 25 years or an older women with risk factors such as new or multiple sex partners, or a sex partner who has a sexually transmitted infection, you should be tested for gonorrhea every year.
I'm pregnant. How does gonorrhea affect my baby?
If you are pregnant and have gonorrhea, you can give the infection to your baby during delivery. This can cause serious health problems for your baby. If you are pregnant, it is important that you talk to your health care provider so that you get the correct examination, testing, and treatment, as necessary. Treating gonorrhea as soon as possible will make health complications for your baby less likely.
How do I know if I have gonorrhea?
Some men with gonorrhea may have no symptoms at all. However, men who do have symptoms, may have:
- A burning sensation when urinating;
- A white, yellow, or green discharge from the penis;
- Painful or swollen testicles (although this is less common).
Most women with gonorrhea do not have any symptoms. Even when a woman has symptoms, they are often mild and can be mistaken for a bladder or vaginal infection. Women with gonorrhea are at risk of developing serious complications from the infection, even if they don’t have any symptoms.
Symptoms in women can include:
- Painful or burning sensation when urinating;
- Increased vaginal discharge;
- Vaginal bleeding between periods.
Rectal infections may either cause no symptoms or cause symptoms in both men and women that may include:
- Discharge;
- Anal itching;
- Soreness;
- Bleeding;
- Painful bowel movements.
You should be examined by your doctor if you notice any of these symptoms or if your partner has an STD or symptoms of an STD, such as an unusual sore, a smelly discharge, burning when urinating, or bleeding between periods.
How will my doctor know if I have gonorrhea?
Most of the time, urine can be used to test for gonorrhea. However, if you have had oral and/or anal sex, swabs may be used to collect samples from your throat and/or rectum. In some cases, a swab may be used to collect a sample from a man’s urethra (urine canal) or a woman’s cervix (opening to the womb).
Antibiotic-Resistant Gonorrhea
Can gonorrhea be cured?
Yes, gonorrhea can be cured with the right treatment. It is important that you take all of the medication your doctor prescribes to cure your infection. Medication for gonorrhea should not be shared with anyone. Although medication will stop the infection, it will not undo any permanent damage caused by the disease.
It is becoming harder to treat some gonorrhea, as drug-resistant strains of gonorrhea are increasing. If your symptoms continue for more than a few days after receiving treatment, you should return to a health care provider to be checked again.
I was treated for gonorrhea. When can I have sex again?
You should wait seven days after finishing all medications before having sex. To avoid getting infected with gonorrhea again or spreading gonorrhea to your partner(s), you and your sex partner(s) should avoid having sex until you have each completed treatment. If you’ve had gonorrhea and took medicine in the past, you can still get infected again if you have unprotected sex with a person who has gonorrhea.
What happens if I don’t get treated?
Untreated gonorrhea can cause serious and permanent health problems in both women and men.
In women, untreated gonorrhea can cause pelvic inflammatory disease (PID). Some of the complications of PID are
- Formation of scar tissue that blocks fallopian tubes;
- Ectopic pregnancy (pregnancy outside the womb);
- Infertility (inability to get pregnant);
- Long-term pelvic/abdominal pain.
In men, gonorrhea can cause a painful condition in the tubes attached to the testicles. In rare cases, this may cause a man to be sterile, or prevent him from being able to father a child.
Rarely, untreated gonorrhea can also spread to your blood or joints. This condition can be life-threatening.
Untreated gonorrhea may also increase your chances of getting or giving HIV – the virus that causes AIDS.
Gonorrhea - CDC Fact Sheet (Detailed Version)
Detailed Version
Detailed fact sheets are intended for physicians and individuals with specific questions about sexually transmitted diseases. Detailed fact sheets include specific testing and treatment recommendations as well as citations so the reader can research the topic more in depth.
What is gonorrhea?
Gonorrhea is a sexually transmitted disease (STD) caused by infection with the Neisseria gonorrhoeae bacterium. N. gonorrhoeae infects the mucous membranes of the reproductive tract, including the cervix, uterus, and fallopian tubes in women, and the urethra in women and men. N. gonorrhoeae can also infect the mucous membranes of the mouth, throat, eyes, and anus.
How common is gonorrhea?
Gonorrhea is a very common infectious disease. CDC estimates that, annually, 820,000 people in the United States get new gonorrheal infections, and less than half of these infections are detected and reported to CDC. CDC estimates that 570,000 of them were among young people 15-24 years of age. In 2013, 333,004 cases of gonorrhea were reported to CDC.2
How do people get gonorrhea?
Gonorrhea is transmitted through sexual contact with the penis, vagina, mouth, or anus of an infected partner. Ejaculation does not have to occur for gonorrhea to be transmitted or acquired. Gonorrhea can also be spread perinatally from mother to baby during childbirth.
People who have had gonorrhea and received treatment may be reinfected if they have sexual contact with a person infected with gonorrhea.
Who is at risk for gonorrhea?
Any sexually active person can be infected with gonorrhea. In the United States, the highest reported rates of infection are among sexually active teenagers, young adults, and African Americans 2.
What are the signs and symptoms of gonorrhea?
Many men with gonorrhea are asymptomatic 3, 4. When present, signs and symptoms of urethral infection in males include dysuria or a white, yellow, or green urethral discharge that usually appears one to fourteen days after infection 5. In cases where urethral infection is complicated by epididymitis, men with gonorrhea may also complain of testicular or scrotal pain.
Most women with gonorrhea are asymptomatic 6, 7. Even when a woman has symptoms, they are often so mild and nonspecific that they are mistaken for a bladder or vaginal infection 8, 9. The initial symptoms and signs in women include dysuria, increased vaginal discharge, or vaginal bleeding between periods. Women with gonorrhea are at risk of developing serious complications from the infection, regardless of the presence or severity of symptoms.
Symptoms of rectal infection in both men and women may include discharge, anal itching, soreness, bleeding, or painful bowel movements 10. Rectal infection also may be asymptomatic. Pharyngeal infection may cause a sore throat, but usually is asymptomatic 11, 12.
What are the complications of gonorrhea?
Untreated gonorrhea can cause serious and permanent health problems in both women and men.
In women, gonorrhea can spread into the uterus or fallopian tubes and cause pelvic inflammatory disease (PID). The symptoms may be quite mild or can be very severe and can include abdominal pain and fever 13. PID can lead to internal abscesses and chronic pelvic pain. PID can also damage the fallopian tubes enough to cause infertility or increase the risk of ectopic pregnancy.
In men, gonorrhea may be complicated by epididymitis. In rare cases, this may lead to infertility 14.
If left untreated, gonorrhea can also spread to the blood and cause disseminated gonococcal infection (DGI). DGI is usually characterized by arthritis, tenosynovitis, and/or dermatitis 15. This condition can be life threatening.
What about Gonorrhea and HIV?
Untreated gonorrhea can increase a person’s risk of acquiring or transmitting HIV, the virus that causes AIDS 16.
How does gonorrhea affect a pregnant woman and her baby?
If a pregnant woman has gonorrhea, she may give the infection to her baby as the baby passes through the birth canal during delivery. This can cause blindness, joint infection, or a life-threatening blood infection in the baby 17. Treatment of gonorrhea as soon as it is detected in pregnant women will reduce the risk of these complications. Pregnant women should consult a health care provider for appropriate examination, testing, and treatment, as necessary.
Who should be tested for gonorrhea?
Any sexually active person can be infected with gonorrhea. Anyone with genital symptoms such as discharge, burning during urination, unusual sores, or rash should stop having sex and see a health care provider immediately.
Also, anyone with an oral, anal, or vaginal sex partner who has been recently diagnosed with an STD should see a health care provider for evaluation.
Some people should be tested (screened) for gonorrhea even if they do not have symptoms or know of a sex partner who has gonorrhea 18. Anyone who is sexually active should discuss his or her risk factors with a health care provider and ask whether he or she should be tested for gonorrhea or other STDs.
CDC recommends yearly gonorrhea screening for all sexually active women younger than 25 years, as well as older women with risk factors such as new or multiple sex partners, or a sex partner who has a sexually transmitted infection.
People who have gonorrhea should also be tested for other STDs.
How is gonorrhea diagnosed?
Urogenital gonorrhea can be diagnosed by testing urine, urethral (for men), or endocervical or vaginal (for women) specimens using nucleic acid amplification testing (NAAT) 19. It can also be diagnosed using gonorrhea culture, which requires endocervical or urethral swab specimens.
If a person has had oral and/or anal sex, pharyngeal and/or rectal swab specimens should be collected either for culture or for NAAT (if the local laboratory has validated the use of NAAT for extra-genital specimens) 20.
What is the treatment for gonorrhea?
Gonorrhea can be cured with the right treatment. CDC now recommends dual therapy (i.e. using two drugs) for the treatment of gonorrhea. It is important to take all of the medication prescribed to cure gonorrhea. Medication for gonorrhea should not be shared with anyone. Although medication will stop the infection, it will not repair any permanent damage done by the disease. Antimicrobial resistance in gonorrhea is of increasing concern, and successful treatment of gonorrhea is becoming more difficult 21. If a person’s symptoms continue for more than a few days after receiving treatment, he or she should return to a health care provider to be reevaluated.
What about partners?
If a person has been diagnosed and treated for gonorrhea, he or she should tell all recent anal, vaginal, or oral sex partners (all sex partners within 60 days before the onset of symptoms or diagnosis) so they can see a health provider and be treated 20. This will reduce the risk that the sex partners will develop serious complications from gonorrhea and will also reduce the person’s risk of becoming reinfected. A person with gonorrhea and all of his or her sex partners must avoid having sex until they have completed their treatment for gonorrhea and until they no longer have symptoms. For tips on talking to partners about sex and STD testing, visit http://www.gytnow.org/talking-to-your-partner.
How can gonorrhea be prevented?
Latex condoms, when used consistently and correctly, can reduce the risk of transmission of gonorrhea. The surest way to avoid transmission of gonorrhea or other STDs is to abstain from sexual intercourse, or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
Antibiotic-Resistant Gonorrhea
The emergence of multidrug- and cephalosporin-resistant gonorrhea in the United States would make gonorrhea much more difficult to treat.
Gonorrhea has progressively developed resistance to the antibiotic drugs prescribed to treat it. Following the spread of gonococcal fluoroquinolone resistance, the cephalosporin antibiotics have been the foundation of recommended treatment for gonorrhea. The emergence of cephalosporin-resistant gonorrhea would significantly complicate the ability of providers to treat gonorrhea successfully, since we have few antibiotic options left that are simple, well-studied, well-tolerated and highly effective. It is critical to continuously monitor antibiotic resistance in Neisseria gonorrhoeae and encourage research and development of new treatment regimens.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
www.cdc.gov/std
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
CDC: Centers for Disease Control and Prevention
back-to-top
Genital Herpes - CDC Fact Sheet

Herpes is a common sexually transmitted disease (STD) that any sexually active person can get. Most people with the virus don’t have symptoms. It is important to know that even without signs of the disease, it can still spread to sexual partners.
Basic Fact Sheet
Basic fact sheets are presented in plain language for individuals with general questions about sexually transmitted diseases. The content here can be syndicated (added to your web site).
What is genital herpes?
Genital herpes is an STD caused by two types of viruses. The viruses are called herpes simplex type 1 and herpes simplex type 2.
How common is genital herpes?
Genital herpes is common in the United States. In the United States, about one out of every six people aged 14 to 49 years have genital herpes.
How is genital herpes spread?
You can get herpes by having vaginal, anal, or oral sex with someone who has the disease.
Fluids found in a herpes sore carry the virus, and contact with those fluids can cause infection. You can also get herpes from an infected sex partner who does not have a visible sore or who may not know he or she is infected because the virus can be released through your skin and spread the infection to your sex partner(s).
How can I reduce my risk of getting herpes?
The only way to avoid STDs is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting herpes:
- Being in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Using latex condoms the right way every time you have sex.
Herpes symptoms can occur in both male and female genital areas that are covered by a latex condom. However, outbreaks can also occur in areas that are not covered by a condom so condoms may not fully protect you from getting herpes.
I'm pregnant. How could genital herpes affect my baby?
If you are pregnant and have genital herpes, it is even more important for you to go to prenatal care visits. You need to tell your doctor if you have ever had symptoms of, been exposed to, or been diagnosed with genital herpes. Sometimes genital herpes infection can lead to miscarriage. It can also make it more likely for you to deliver your baby too early. Herpes infection can be passed from you to your unborn child and cause a potentially deadly infection (neonatal herpes). It is important that you avoid getting herpes during pregnancy.
If you are pregnant and have genital herpes, you may be offered herpes medicine towards the end of your pregnancy to reduce the risk of having any symptoms and passing the disease to your baby. At the time of delivery your doctor should carefully examine you for symptoms. If you have herpes symptoms at delivery, a ‘C-section’ is usually performed.
How do I know if I have genital herpes?
Most people who have herpes have no, or very mild symptoms. You may not notice mild symptoms or you may mistake them for another skin condition, such as a pimple or ingrown hair. Because of this, most people who have herpes do not know it.
Genital herpes sores usually appear as one or more blisters on or around the genitals, rectum or mouth. The blisters break and leave painful sores that may take weeks to heal. These symptoms are sometimes called “having an outbreak.” The first time someone has an outbreak they may also have flu-like symptoms such as fever, body aches, or swollen glands.
Repeat outbreaks of genital herpes are common, especially during the first year after infection. Repeat outbreaks are usually shorter and less severe than the first outbreak. Although the infection can stay in the body for the rest of your life, the number of outbreaks tends to decrease over a period of years.
You should be examined by your doctor if you notice any of these symptoms or if your partner has an STD or symptoms of an STD, such as an unusual sore, a smelly discharge, burning when urinating, or, for women specifically, bleeding between periods.
How will my doctor know if I have herpes?
Often times, your healthcare provider can diagnose genital herpes by simply looking at your symptoms. Providers can also take a sample from the sore(s) and test it. Have an honest and open talk with your health care provider and ask whether you should be tested for herpes or other STDs.
Can herpes be cured?
There is no cure for herpes. However, there are medicines that can prevent or shorten outbreaks. One of these herpes medicines can be taken daily, and makes it less likely that you will pass the infection on to your sex partner(s).
What happens if I don't get treated?
Genital herpes can cause painful genital sores and can be severe in people with suppressed immune systems. If you touch your sores or the fluids from the sores, you may transfer herpes to another part of your body, such as your eyes. Do not touch the sores or fluids to avoid spreading herpes to another part of your body. If you touch the sores or fluids, immediately wash your hands thoroughly to help avoid spreading your infection.
Some people who get genital herpes have concerns about how it will impact their overall health, sex life, and relationships. It is best for you to talk to a health care provider about those concerns, but it also is important to recognize that while herpes is not curable, it can be managed. Since a genital herpes diagnosis may affect how you will feel about current or future sexual relationships, it is important to understand how to talk to sexual partners about STDs. You can find one resource here: GYT Campaign.
If you are pregnant, there can be problems for you and your unborn child. See “I’m pregnant. How could genital herpes affect my baby?” above for information about this.
Can I still have sex if I have herpes?
If you have herpes, you should tell your sex partner(s) and let him or her know that you do and the risk involved. Using condoms may help lower this risk but it will not get rid of the risk completely. Having sores or other symptoms of herpes can increase your risk of spreading the disease. Even if you do not have any symptoms, you can still infect your sex partners.
What is the link between genital herpes and HIV?
Genital herpes can cause sores or breaks in the skin or lining of the mouth, vagina, and rectum. The genital sores caused by herpes can bleed easily. When the sores come into contact with the mouth, vagina, or rectum during sex, they increase the risk of giving or getting HIV if you or your partner has HIV.
Genital Herpes - CDC Fact Sheet (Detailed)
Detailed Version
Detailed fact sheets are intended for physicians and individuals with specific questions about sexually transmitted diseases. Detailed fact sheets include specific testing and treatment recommendations as well as citations so the reader can research the topic more in depth.
What is genital herpes?
Genital herpes is a sexually transmitted disease (STD) caused by the herpes simplex viruses type 1 (HSV-1) or type 2 (HSV-2).
How common is genital herpes?
CDC estimates that, annually, 776,000 people in the United States get new herpes infections. Genital herpes infection is common in the United States. Nationwide, 15.5 % of persons aged 14 to 49 years have HSV-2 infection. The overall prevalence of genital herpes is likely higher than 15.5%, because an increasing number of genital herpes infections are caused by HSV-1. HSV-1 is typically acquired in childhood; as the prevalence of HSV-1 infection has declined in recent decades, people may have become more susceptible to genital herpes from HSV-1.
HSV-2 infection is more common among women than among men (20.3% versus 10.6% in 14 to 49 year olds). Infection is more easily transmitted from men to women than from women to men. HSV-2 infection is more common among non-Hispanic blacks (41.8%) than among non-Hispanic whites (11.3%). This disparity remains even among persons with similar numbers of lifetime sexual partners. For example, among persons with 2–4 lifetime sexual partners, HSV-2 is still more prevalent among non-Hispanic blacks (34.3%) than among non-Hispanic whites (9.1%) or Mexican Americans (13%). Most infected persons are unaware of their infection. In the United States, an estimated 87.4% of 14–49 year olds infected with HSV-2 have never received a clinical diagnosis.
The percentage of persons in the United States who are infected with HSV-2 decreased from 21.2% in 1988–1994 to 15.5% in 2007-2010.
How do people get genital herpes?
Infections are transmitted through contact with lesions, mucosal surfaces, genital secretions, or oral secretions. HSV-1 and HSV-2 can also be shed from skin that looks normal. Generally, a person can only get HSV-2 infection during sexual contact with someone who has a genital HSV-2 infection. Transmission most commonly occurs from an infected partner who does not have a visible sore and may not know that he or she is infected. In persons with asymptomatic HSV-2 infections, genital HSV shedding occurs on 10% of days, and on most of those days the person has no signs or symptoms.
What are the symptoms of genital herpes?
Most individuals infected with HSV-1 or HSV-2 are asymptomatic, or have very mild symptoms that go unnoticed or are mistaken for another skin condition. As a result, 87.4% of infected individuals remain unaware of their infection.When symptoms do occur, they typically appear as one or more vesicles on or around the genitals, rectum or mouth. The average incubation period after exposure is 4 days (range, 2 to 12). 5 The vesicles break and leave painful ulcers that may take two to four weeks to heal. Experiencing these symptoms is referred to as having an "outbreak," or episode.
Clinical manifestations of genital herpes differ between the first and recurrent outbreaks of HSV. The first outbreak of herpes is often associated with a longer duration of herpetic lesions, increased viral shedding (making HSV transmission more likely) and systemic symptoms including fever, body aches, swollen lymph nodes, and headache. Recurrent outbreaks of genital herpes are common, in particular during the first year of infection. Approximately half of patients who recognize recurrences have prodromal symptoms, such as mild tingling or shooting pains in the legs, hips and buttocks occurring hours to days before eruption of herpetic lesions. Symptoms of recurrent outbreaks are typically shorter in duration and less severe than the first outbreak of genital herpes. Although the infection can stay in the body indefinitely, the number of outbreaks tends to decrease over time. Recurrences are much less frequent for genital HSV-1 infection than for genital HSV-2 infection.
What are the complications of genital herpes?
Genital herpes causes painful genital ulcers in many adults that can be severe and persistent in persons with suppressed immune systems, such as HIV-infected persons. Both HSV-1 and HSV-2 can also cause rare but serious complications such as blindness, encephalitis (inflammation of the brain), and aseptic meningitis (inflammation of the linings of the brain). Development of extragenital lesions in the buttocks, groin, thigh, finger, and eye may occur during the course of infection.
Some persons who contract genital herpes have concerns about how it will impact their overall health, sex life, and relationships. There can be can be considerable embarrassment, shame, and stigma associated with a herpes diagnosis and this can substantially interfere with a patient’s relationships. Clinicians can address these concerns by encouraging patients to recognize that while herpes is not curable, it is a manageable condition. Three important steps that providers can take for their newly-diagnosed patients are: giving information, providing support resources, and helping define options. 8 Since a genital herpes diagnosis may affect perceptions about existing or future sexual relationships, it is important for patients to understand how to talk to sexual partners about STDs. One resource can be found here: www.gytnow.org/talking-to-your-partner
There are also potential complications for a pregnant woman and her unborn child. See “How does herpes infection affect a pregnant woman and her baby?” below for information about this.
HIV/AIDS & STDs
What is the link between genital herpes and HIV?
Genital ulcerative disease caused by herpes make it easier to transmit and acquire HIV infection sexually. There is an estimated 2- to 4-fold increased risk of acquiring HIV, if exposed to HIV when genital herpes is present. This is because genital herpes can cause ulcers or breaks in the skin or mucous membranes (lining of the mouth, vagina, and rectum), which compromises the protection normally provided by the skin and mucous membranes against infections, including HIV. Herpetic genital ulcers can bleed easily, and when they come into contact with the mouth, vagina, or rectum during sex, they increase the risk of HIV transmission.
How does genital herpes affect a pregnant woman and her baby?
Neonatal herpes is one of the most serious complications of genital herpes. Healthcare providers should ask all pregnant women if they have a history of genital herpes. Herpes infection can be passed from mother to child during pregnancy, childbirth, or in the newborn period, resulting in a potentially fatal neonatal herpes infection. During pregnancy there is a higher risk of perinatal transmission during the first outbreak than with a recurrent outbreak, thus it is important that women avoid contracting herpes during pregnancy. Women should be counseled to abstain from intercourse during the third trimester with partners known to have or suspected of having genital herpes.
A woman with genital herpes may be offered antiviral medication from 36 weeks gestation through delivery to reduce the risk of a recurrent outbreak. Routine HSV screening of pregnant women is not recommended. However, at onset of labor, all women should undergo careful examination and questioning to evaluate for presence of prodromal symptoms or herpetic lesions. If herpes symptoms are present a cesarean delivery is recommended to prevent HSV transmission to the infant.
How is genital herpes diagnosed?
Numerous herpes diagnostic tests are available. Direct (or virologic) tests detect viable virus, viral antigen, or viral nucleic acid. Viral culture is currently the reference standard for diagnosing genital herpes. HSV culture requires collection of a sample from the sore and, once viral growth is seen, specific cell staining to differentiate between HSV-1 and HSV-2. Nucleic acid amplification techniques (NAATs), such as PCR, test for viral DNA or RNA and allow for more rapid and accurate results. Indirect (or serologic) tests are blood tests that detect antibodies to the herpes virus. Several ELISA-based serologic tests are FDA approved and available commercially. Older assays that do not accurately distinguish HSV-1 from HSV-2 antibody remain on the market, so providers should specifically request serologic type-specific assays when blood tests are performed for their patients. HSV-1 ELISA results are considered to be reliable because HSV-1 is ubiquitous in most populations. However, false positive HSV-2 ELISA results are more often seen when testing is done in populations with a lower prevalence of HSV-2.
For the symptomatic patient, testing with both direct and indirect assays can determine whether it is a new infection or a newly-recognized old infection. A primary infection would be supported by a positive virologic test and a negative serologic test, while the diagnosis of recurrent disease would be supported by positive virologic and serologic test results.
CDC does not recommend screening for HSV-1 or HSV-2 in the general population. Several scenarios where type-specific HSV tests may be useful include
- Patients with recurrent genital symptoms or atypical symptoms and negative HSV cultures;
- Patients with a clinical diagnosis of genital herpes but no laboratory confirmation;
- Patients who report having a partner with genital herpes;
- Patients presenting for an STD evaluation (especially those with multiple partners);
- Persons with HIV infection; and
- MSM at increased risk for HIV acquisition.
Is there a cure or treatment for herpes?
There is no cure for herpes. Antiviral medications can, however, prevent or shorten outbreaks during the period of time the person takes the medication. In addition, daily suppressive therapy (i.e. daily use of antiviral medication) for herpes can reduce the likelihood of transmission to partners.
Several clinical trials have tested vaccines against genital herpes infection, but there is currently no commercially available vaccine that is protective against genital herpes infection. One vaccine trial showed efficacy among women whose partners were HSV-2 infected, but only among women who were not infected with HSV-1. No efficacy was observed among men whose partners were HSV-2 infected. A subsequent trial testing the same vaccine showed some protection from genital HSV-1 infection, but no protection from HSV-2 infection.
How can herpes be prevented?
Correct and consistent use of latex condoms can reduce the risk of genital herpes. However, outbreaks can occur in areas that are not covered by a condom.
The surest way to avoid transmission of sexually transmitted diseases, including genital herpes, is to abstain from sexual contact, or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
Persons with herpes should abstain from sexual activity with partners when sores or other symptoms of herpes are present. It is important to know that even if a person does not have any symptoms, he or she can still infect sex partners. Sex partners of infected persons should be advised that they may become infected and they should use condoms to reduce the risk. Sex partners can seek testing to determine if they are infected with HSV.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
Personal health inquiries and information about STDs:
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
Resources:
CDC National Prevention Information Network (NPIN)
P.O. Box 6003
Rockville, MD 20849-6003
E-mail: npin-info@cdc.gov
Source: Centers for Disease Control and Prevention. HHS
back-to-top
STDs and HIV – CDC Fact Sheet

People who have STDs are more likely to get HIV, when compared to people who do not have STDs.
Basic Fact Sheet
Basic fact sheets are presented in plain language for individuals with general questions about sexually transmitted diseases.
Are some STDs associated with HIV?
Yes. In the United States, people who get syphilis, gonorrhea, and herpes often also have HIV, or are more likely to get HIV in the future.
Why does having an STD put me more at risk for getting HIV?
If you get an STD you are more likely to get HIV than someone who is STD-free. This is because the same behaviors and circumstances that may put you at risk for getting an STD can also put you at greater risk for getting HIV. In addition, having a sore or break in the skin from an STD may allow HIV to more easily enter your body.
What activities can put me at risk for both STDs and HIV?
- Having anal, vaginal, or oral sex without a condom;
- Having multiple sex partners;
- Having anonymous sex partners;
- Having sex while under the influence of drugs or alcohol can lower inhibitions and result in greater sexual risk-taking.
What can I do to prevent getting STDs and HIV?
The only way to avoid STDs is to not have vaginal, anal, or oral sex. If you are sexually active, you can do the following things to lower your chances of getting STDs and HIV:
- Choose less risky sexual behaviors
- Use condoms consistently and correctly;
- Reduce the number of people with whom you have sex;
- Limit or eliminate drug and alcohol use before and during sex;
- Have an honest and open talk with your healthcare provider and ask whether you should be tested for STDs and HIV;
- Talk to your healthcare provider and find out if pre-exposure prophylaxis, or PrEP, is a good option for you to prevent HIV infection.
If I already have HIV, and then I get an STD, does that put my sex partner(s) at an increased risk for getting HIV?
It can. If you already have HIV, and then get another STD, it can put your HIV-negative partners at greater risk of getting HIV from you.
Your sex partners are less likely to get HIV from you if you
- Use antiretroviral therapy (ART). ART reduces the amount of virus (viral load) in your blood and body fluids. ART can keep you healthy for many years, and greatly reduce your chance of transmitting HIV to sex partners, if taken consistently.
- Choose less risky sexual behaviors.
- Use condoms consistently and correctly.
The risk of getting HIV may also be reduced if your partner takes pre-exposure prophylaxis, or PrEP, after discussing this option with his or her healthcare provider and determining whether it is appropriate.
Will treating STDs prevent me from getting HIV?
No. It’s not enough.
If you get treated for an STD, this will help to prevent its complications, and prevent spreading STDs to your sex partners. Treatment for an STD other than HIV does not prevent the spread of HIV.
If you are diagnosed with an STD, talk to your doctor about ways to protect yourself and your partner(s) from getting reinfected with the same STD, or getting HIV.
STDs and HIV – CDC Fact Sheet
People who have STDs are more likely to get HIV, when compared to people who do not have STDs.
Detailed Version
Detailed fact sheets are intended for physicians and individuals with specific questions about sexually transmitted diseases. Detailed fact sheets include specific testing and treatment recommendations as well as citations so the reader can research the topic more in depth.
Are STDs related to HIV?
Yes. In the United States, people who get syphilis, gonorrhea, and herpes often also have HIV or are more likely to get HIV in the future. One reason is the behaviors that put someone at risk for one infection (not using condoms, multiple partners, anonymous partners) often put them at risk for other infections. Also, because STD and HIV tend to be linked, when someone gets an STD it suggests they got it from someone who may be at risk for other STD and HIV. Finally, a sore or inflammation from an STD may allow infection with HIV that would have been stopped by intact skin.
STDs can increase the risk of spreading HIV.
HIV-infected persons are more likely to shed HIV when they have urethritis or a genital ulcer. When HIV-infected persons get another STD such as gonorrhea or syphilis, it suggests that they were having sex without using condoms. If so, they may have spread HIV to their partners.
Some STDs are more closely linked to HIV than others.
In the US, both syphilis and HIV are highly concentrated epidemics among men who have sex with men. In 2012, approximately 75% of persons reported with syphilis in the U.S. were MSM. In Florida, in 2010, among all persons diagnosed with infectious syphilis 42% were also HIV infected. Men who get syphilis are at very high risk of being diagnosed with HIV in the future; among HIV-uninfected men who got syphilis in Florida in 2003, 22% were newly diagnosed with HIV by 2011. HIV is more closely linked to gonorrhea than chlamydia (which is particularly common among young women). Herpes is also commonly associated with HIV; a meta-analysis found persons infected with HSV-2 are at 3-fold increased risk for acquiring HIV infection.
Some activities can put people at increased risk for both STDs and HIV.
- Having anal, vaginal, or oral sex without a condom;
- Having multiple sex partners;
- Having anonymous sex partners;
- Having sex while under the influence of drugs or alcohol can lower inhibitions and result in greater sexual risk taking.
Does treating STDs prevent HIV?
Not by itself. Given the close link between STD and HIV in many studies, it seems obvious that treating STDs should reduce the risk of HIV. However, studies that have lowered the risk of STD in communities have not necessarily lowered the risk of HIV. Risk of HIV was lowered in one community trial, but not in 3 others.
- In Mwanza (Tanzania), improved STD treatment lowered 2-year HIV incidence by 40% in the intervention towns (1.2%) compared to other towns (1.9%).
- In Rakai (Uganda), a more intensive intervention (mass treatment and improved STD control) was done, leading to lower rates of syphilis and trichomoniasis, but the incidence of HIV was the same in intervention and comparison towns (1.5% per year).
- A third community trial found no difference in HIV incidence when behavioral plus STD control interventions were compared to usual services (Incidence rate ratio = 1.00), despite lower rates of syphilis (rate ratio 0.52) and gonorrhea (rate ratio 0.25).
- A fourth community trial found HIV incidence was slightly higher in communities that received a combination of interventions including improved STD treatment when compared to control communities (incidence rate ratio 1.27, not statistically significant).
Treating individuals for STDs has also not necessarily lowered their risk of acquiring HIV.
- One study found there was slightly lower risk of HIV seroconversion among female sex workers who had monthly exams for STD (5.3%) compared to sex workers who were examined when they had symptoms (7.6%, P=0.5); their rates of infection were lower for trichomonas (14% vs 7% P=0.07) but not for gonorrhea, chlamydia, or genital ulcers.
- A second trial in female sex workers found a slightly higher incidence of HIV among women who received monthly treatment with azithromycin (4%) compared to women who did not (3.2%, P=0.5) despite major differences in the incidence of infection with gonorrhea (relative risk RR 0.46), chlamydia (RR 0.38), and trichomoniasis (RR 0.56).
Three placebo-controlled trials have assessed the benefit to individuals from treatment with acyclovir to suppress genital herpes ulcers:
- One enrolled female sex workers who were infected with HSV but not HIV; it found no impact on HIV incidence in the acyclovir group (4.29%) compared to the placebo group (4.25%), though it also found no difference in reported episodes of genital ulceration or in measured HSV shedding.
- A second study of HIV acquisition among persons infected with HSV-2 included women and men who have sex with men; HIV incidence was similar in the acyclovir group (3.9%) and the placebo group (3.3%) despite a 47% reduction in observed genital ulcers in the acyclovir group.
- The third study looked at the effect of acyclovir on HIV transmission from heterosexuals infected with both HIV and HSV-2 to their HIV-uninfected partners; after removing 29% of new infections that were apparently acquired from an outside partner, the incidence was similar in the acyclovir group (1.8%) and the placebo group (1.9%, P=0.69) despite major reductions in genital ulcer disease (risk ratio 0.39).
Screening for STDs can help assess a person’s risk for getting HIV. Treatment of STDs is important to prevent the complications of those infections, and to prevent transmission to partners, but it should not be expected to prevent spread of HIV.
What can people do to reduce their risk of getting STDs and HIV?
The only way to avoid STDs is to not have vaginal, anal, or oral sex. If people are sexually active, they can do the following things to lower their chances of getting STDs and HIV:
- Choose less risky sexual behaviors;
- Use condoms consistently and correctly;
- Reduce the number of people with whom they have sex;
- Limit or eliminate drug and alcohol use before and during sex;
- Have an honest and open talk with their healthcare provider and ask whether they should be tested for STDs and HIV.
- Talk with their healthcare provider and find out if pre-exposure prophylaxis, or PrEP, is a good option for them to prevent HIV infection.
If someone already has HIV, and subsequently gets an STD, does that put their sex partner(s) at an increased risk for getting HIV?
It can. HIV-negative sex partners are at greater risk of getting HIV from someone who is HIV-positive and acquires another STD. The HIV-negative sex partners of persons who are HIV-positive are less likely to get HIV if:
- HIV-positive persons use antiretroviral therapy (ART). ART reduces the amount of virus (viral load) in blood and body fluids. ART can keep HIV-positive persons healthy for many years, and greatly reduce the chance of transmitting HIV to sex partners if taken consistently.
- Sex partners take pre-exposure prophylaxis (PrEP) after discussing this option with his/her healthcare provider and determining whether it is appropriate.
- Choose less risky sexual behaviors.
- Use condoms consistently and correctly.
Will treating someone for STDs prevent them from getting HIV?
No. It’s not enough. Screening for STDs can help assess a person’s risk for getting HIV. Treatment of STDs is important to prevent the complications of those infections, and to prevent transmission to partners, but it should not be expected to prevent spread of HIV.
If someone HIV-positive is diagnosed with an STD, they should receive counseling about risk reduction and how to protect their sex partner(s) from getting re-infected with the same STD or getting HIV.
Where can I get more information?
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
CDC National Prevention Information Network (NPIN)
P.O. Box 6003
Rockville, MD 20849-6003
E-mail: npin-info@cdc.gov
CDC: Centers for Disease Control and Prevention. HHS
back-to-top
Genital HPV Infection - Fact Sheet

Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States. Some health effects caused by HPV can be prevented with vaccines.
What is HPV?
HPV is the most common sexually transmitted infection (STI). HPV is a different virus than HIV and HSV (herpes). HPV is so common that nearly all sexually active men and women get it at some point in their lives. There are many different types of HPV. Some types can cause health problems including genital warts and cancers. But there are vaccines that can stop these health problems from happening.
How is HPV spread?
You can get HPV by having vaginal, anal, or oral sex with someone who has the virus. It is most commonly spread during vaginal or anal sex. HPV can be passed even when an infected person has no signs or symptoms.
Anyone who is sexually active can get HPV, even if you have had sex with only one person. You also can develop symptoms years after you have sex with someone who is infected making it hard to know when you first became infected.
Does HPV cause health problems?
In most cases, HPV goes away on its own and does not cause any health problems. But when HPV does not go away, it can cause health problems like genital warts and cancer.
Genital warts usually appear as a small bump or group of bumps in the genital area. They can be small or large, raised or flat, or shaped like a cauliflower. A healthcare provider can usually diagnose warts by looking at the genital area.
Does HPV cause cancer?
HPV can cause cervical and other cancers including cancer of the vulva, vagina, penis, or anus. It can also cause cancer in the back of the throat, including the base of the tongue and tonsils (called oropharyngeal cancer).
Cancer often takes years, even decades, to develop after a person gets HPV. The types of HPV that can cause genital warts are not the same as the types of HPV that can cause cancers.
There is no way to know which people who have HPV will develop cancer or other health problems. People with weak immune systems (including individuals with HIV/AIDS) may be less able to fight off HPV and more likely to develop health problems from it.
How can I avoid HPV and the health problems it can cause?
You can do several things to lower your chances of getting HPV.
Get vaccinated. HPV vaccines are safe and effective. They can protect males and females against diseases (including cancers) caused by HPV when given in the recommended age groups (see “Who should get vaccinated?” below). HPV vaccines are given in three shots over six months; it is important to get all three doses.
Get screened for cervical cancer. Routine screening for women aged 21 to 65 years old can prevent cervical cancer.
If you are sexually active
- Use latex condoms the right way every time you have sex. This can lower your chances of getting HPV. But HPV can infect areas that are not covered by a condom - so condoms may not give full protection against getting HPV;
- Be in a mutually monogamous relationship – or have sex only with someone who only has sex with you.
Who should get vaccinated?
All boys and girls ages 11 or 12 years should get vaccinated.
Catch-up vaccines are recommended for males through age 21 and for females through age 26, if they did not get vaccinated when they were younger.
The vaccine is also recommended for gay and bisexual men (or any man who has sex with a man) through age 26. It is also recommended for men and women with compromised immune systems (including people living with HIV/AIDS) through age 26, if they did not get fully vaccinated when they were younger.
How do I know if I have HPV?
There is no test to find out a person’s “HPV status.” Also, there is no approved HPV test to find HPV in the mouth or throat.
There are HPV tests that can be used to screen for cervical cancer. These tests are recommended for screening only in women aged 30 years and older. They are not recommended to screen men, adolescents, or women under the age of 30 years.
Most people with HPV do not know they are infected and never develop symptoms or health problems from it. Some people find out they have HPV when they get genital warts. Women may find out they have HPV when they get an abnormal Pap test result (during cervical cancer screening). Others may only find out once they’ve developed more serious problems from HPV, such as cancers.
How common is HPV and the health problems caused by HPV?
HPV (the virus): About 79 million Americans are currently infected with HPV. About 14 million people become newly infected each year. HPV is so common that most sexually-active men and women will get at least one type of HPV at some point in their lives.
Health problems related to HPV include genital warts and cervical cancer.
Genital warts: About 360,000 people in the United States get genital warts each year.
Cervical cancer: More than 11,000 women in the United States get cervical cancer each year.
There are other conditions and cancers caused by HPV that occur in persons living in the United States.
I'm pregnant. Will having HPV affect my pregnancy?
If you are pregnant and have HPV, you can get genital warts or develop abnormal cell changes on your cervix. Abnormal cell changes can be found with routine cervical cancer screening. You should get routine cervical cancer screening even when you are pregnant.
Can I be treated for HPV or health problems caused by HPV?
There is no treatment for the virus itself. However, there are treatments for the health problems that HPV can cause:
- Genital warts can be treated by you or your physician. If left untreated, genital warts may go away, stay the same, or grow in size or number.
- Cervical precancer can be treated. Women who get routine Pap tests and follow up as needed can identify problems before cancer develops. Prevention is always better than treatment. For more information visit www.cancer.org.
- Other HPV-related cancer are also more treatable when diagnosed and treated early. For more information visit www.cancer.org. All STD Fact Sheets
Where can I get more information?
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
CDC National Prevention Information Network (NPIN)
P.O. Box 6003
Rockville, MD 20849-6003
E-mail: npin-info@cdc.gov
National HPV and Cervical Cancer Prevention Resource Center American Sexual Health Association (ASHA)
P. O. Box 13827
Research Triangle Park, NC
27709-3827
1-800-783-9877
CDC: Centers for Disease Control and Prevention, HHS
back-to-top
Syphilis - CDC Fact Sheet

Syphilis is a sexually transmitted disease (STD) that can have very serious complications when left untreated, but it is simple to cure with the right treatment.
Basic Fact Sheet | Detailed Version | View Images of Symptoms
Basic fact sheets are presented in plain language for individuals with general questions about sexually transmitted diseases.
The content here can be syndicated (added to your web site).
Print Version
Commercial Print Version
What is syphilis?
Syphilis is an STD that can cause long-term complications if not treated correctly. Symptoms in adults are divided into stages. These stages are primary, secondary, latent, and late syphilis.
How is syphilis spread?
You can get syphilis by direct contact with a syphilis sore during vaginal, anal, or oral sex. Sores can be found on the penis, vagina, anus, in the rectum, or on the lips and in the mouth. Syphilis can also be spread from an infected mother to her unborn baby.
What does syphilis look like?
Syphilis has been called ‘the great imitator’ because it has so many possible symptoms, many of which look like symptoms from other diseases. The painless syphilis sore that you would get after you are first infected can be confused for an ingrown hair, zipper cut, or other seemingly harmless bump. The non-itchy body rash that develops during the second stage of syphilis can show up on the palms of your hands and soles of your feet, all over your body, or in just a few places. You could also be infected with syphilis and have very mild symptoms or none at all.
Example of a primary syphilis sore.
How can I reduce my risk of getting syphilis?
The only way to avoid STDs is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting syphilis:
- Being in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Using latex condoms the right way every time you have sex. Condoms prevent transmission of syphilis by preventing contact with a sore. Sometimes sores occur in areas not covered by a condom. Contact with these sores can still transmit syphilis.
Am I at risk for syphilis?
Any sexually active person can get syphilis through unprotected vaginal, anal, or oral sex. Have an honest and open talk with your health care provider and ask whether you should be tested for syphilis or other STDs. You should get tested regularly for syphilis if you are pregnant, are a man who has sex with men, have HIV infection, and/or have partner(s) who have tested positive for syphilis.
I’m pregnant. How does syphilis affect my baby?
If you are pregnant and have syphilis, you can give the infection to your unborn baby. Having syphilis can lead to a low birth weight baby. It can also make it more likely you will deliver your baby too early or stillborn (a baby born dead). To protect your baby, you should be tested for syphilis during your pregnancy and at delivery and receive immediate treatment if you test positive.
An infected baby may be born without signs or symptoms of disease. However, if not treated immediately, the baby may develop serious problems within a few weeks. Untreated babies can have health problems such as cataracts, deafness, or seizures, and can die.
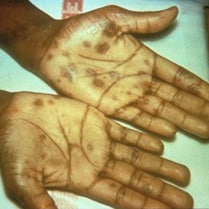
Secondary rash from syphilis on palms of hands.
How do I know if I have syphilis?
Symptoms of syphilis in adults can be divided into stages:
Primary Stage
During the first (primary) stage of syphilis, you may notice a single sore, but there may be multiple sores. The sore is the location where syphilis entered your body. The sore is usually firm, round, and painless. Because the sore is painless, it can easily go unnoticed. The sore lasts 3 to 6 weeks and heals regardless of whether or not you receive treatment. Even though the sore goes away, you must still receive treatment so your infection does not move to the secondary stage.
Secondary Stage
During the secondary stage, you may have skin rashes and/or sores in your mouth, vagina, or anus (also called mucous membrane lesions). This stage usually starts with a rash on one or more areas of your body. The rash can show up when your primary sore is healing or several weeks after the sore has healed. The rash can look like rough, red, or reddish brown spots on the palms of your hands and/or the bottoms of your feet. The rash usually won’t itch and it is sometimes so faint that you won’t notice it. Other symptoms you may have can include fever, swollen lymph glands, sore throat, patchy hair loss, headaches, weight loss, muscle aches, and fatigue (feeling very tired). The symptoms from this stage will go away whether or not you receive treatment. Without the right treatment, your infection will move to the latent and possibly late stages of syphilis.
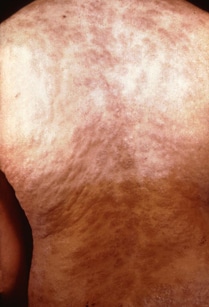
Secondary rash from syphilis on torso.
Latent and Late Stages
The latent stage of syphilis begins when all of the symptoms you had earlier disappear. If you do not receive treatment, you can continue to have syphilis in your body for years without any signs or symptoms. Most people with untreated syphilis do not develop late stage syphilis. However, when it does happen it is very serious and would occur 10–30 years after your infection began. Symptoms of the late stage of syphilis include difficulty coordinating your muscle movements, paralysis (not able to move certain parts of your body), numbness, blindness, and dementia (mental disorder). In the late stages of syphilis, the disease damages your internal organs and can result in death.
A syphilis infection is called an ‘early’ case if a patient has been infected for a year or less, such as during the primary or secondary stages of syphilis. People who have ‘early’ syphilis infections can more easily spread the infection to their sex partners. The majority of early syphilis cases are currently found among men who have sex with men, but women and unborn children are also at risk of infection.
How will my doctor know if I have syphilis?
Most of the time, a blood test can be used to test for syphilis. Some health care providers will diagnose syphilis by testing fluid from a syphilis sore.
Can syphilis be cured?

Darkfield micrograph of Treponema pallidum.
Yes, syphilis can be cured with the right antibiotics from your health care provider. However, treatment will not undo any damage that the infection has already done.
I’ve been treated. Can I get syphilis again?
Having syphilis once does not protect you from getting it again. Even after you’ve been successfully treated, you can still be re-infected. Only laboratory tests can confirm whether you have syphilis. Follow-up testing by your health care provider is recommended to make sure that your treatment was successful.
Because syphilis sores can be hidden in the vagina, anus, under the foreskin of the penis, or in the mouth, it may not be obvious that a sex partner has syphilis. Unless you know that your sex partner(s) has been tested and treated, you may be at risk of getting syphilis again from an untreated sex partner.
Basic Fact Sheet | Detailed Version | View Images of Symptoms
Detailed fact sheets are intended for physicians and individuals with specific questions about sexually transmitted diseases. Detailed fact sheets include specific testing and treatment recommendations as well as citations so the reader can research the topic more in depth.
What is syphilis?
Syphilis is a sexually transmitted disease (STD) caused by the bacterium Treponema pallidum. Syphilis can cause long-term complications if not adequately treated.
How common is syphilis?
During 2013, there were 56,471 reported new cases of syphilis, compared to 48,893 estimated new diagnoses of HIV infection in 2012 and 333,004 cases of gonorrhea in 2013. Of syphilis cases, 17,535 were of primary and secondary (P&S) syphilis, the earliest and most transmissible stages of syphilis. During the 1990s, syphilis primarily occurred among heterosexual men and women of racial and ethnic minority groups; during the 2000s, however, cases increased among men who have sex with men (MSM). 1 In 2002, rates of P&S syphilis were highest among men 30–39 years-old, but in 2013, were highest among men 20–29 years-old. 2, 3 This epidemiologic shift reflects increasing cases reported among young MSM in recent years. 4 MSM accounted for 75% of all P&S syphilis cases in 2013.
Black, Hispanic, and other racial/ethnic minorities are disproportionately affected by P&S syphilis in the United States, with black Americans accounting for most of P&S syphilis among individuals who are not MSM. 3
While the rate of congenital syphilis (syphilis passed from pregnant women to their babies) has decreased in recent years, 3 more cases of congenital syphilis are reported in the United States than cases of perinatal HIV infection. During 2013, 350 cases of congenital syphilis were reported, compared to an estimated 162 cases of perinatal HIV infection during 2010. 5 Congenital syphilis rates were 10.4 times and 3.5 times higher among infants born to black and Hispanic mothers (29.0 and 9.7 cases per 100,000 live births, respectively) compared to white mothers (2.8 cases per 100,000 live births). 6
How do people get syphilis?
Syphilis is transmitted from person to person by direct contact with a syphilitic sore, known as a chancre. Chancres occur mainly on the external genitals, vagina, anus, or in the rectum. Chancres also can occur on the lips and in the mouth. Transmission of syphilis occurs during vaginal, anal, or oral sex. Pregnant women with the disease can transmit it to their unborn child.
How quickly do symptoms appear after infection?
The average time between infection with syphilis and the start of the first symptom is 21 days, but can range from 10 to 90 days.
What are the signs and symptoms in adults?
Syphilis has been called “The Great Pretender”, as its symptoms can look like many other diseases. However, syphilis typically follows a progression of stages that can last for weeks, months, or even years:
Primary Stage
The appearance of a single chancre marks the primary (first) stage of syphilis symptoms, but there may be multiple sores. The chancre is usually firm, round, and painless. It appears at the location where syphilis entered the body. Possibly because these painless chancres can occur in locations that make them difficult to find (e.g., the vagina or anus), smaller proportions of MSM and women are diagnosed in primary stage than men having sex with women only. 3 The chancre lasts 3 to 6 weeks and heals regardless of whether a person is treated or not. However, if the infected person does not receive adequate treatment, the infection progresses to the secondary stage.
Secondary Stage
Skin rashes and/or mucous membrane lesions (sores in the mouth, vagina, or anus) mark the second stage of symptoms. This stage typically starts with the development of a rash on one or more areas of the body. Rashes associated with secondary syphilis can appear when the primary chancre is healing or several weeks after the chancre has healed. The rash usually does not cause itching. The characteristic rash of secondary syphilis may appear as rough, red, or reddish brown spots both on the palms of the hands and the bottoms of the feet. However, rashes with a different appearance may occur on other parts of the body, sometimes resembling rashes caused by other diseases. Sometimes rashes associated with secondary syphilis are so faint that they are not noticed. Large, raised, gray or white lesions, known as condyloma lata, may develop in warm, moist areas such as the mouth, underarm or groin region. In addition to rashes, symptoms of secondary syphilis may include fever, swollen lymph glands, sore throat, patchy hair loss, headaches, weight loss, muscle aches, and fatigue. The symptoms of secondary syphilis will go away with or without treatment, but without treatment, the infection will progress to the latent and possibly late stages of disease.
Latent and Late Stages
The latent (hidden) stage of syphilis begins when primary and secondary symptoms disappear. Without treatment, the infected person will continue to have syphilis infection in their body even though there are no signs or symptoms. Early latent syphilis is latent syphilis where infection occurred within the past 12 months. Late latent syphilis is latent syphilis where infection occurred more than 12 months ago. Latent syphilis can last for years.
The late stages of syphilis can develop in about 15% of people who have not been treated for syphilis, and can appear 10–20 years after infection was first acquired. In the late stages of syphilis, the disease may damage the internal organs, including the brain, nerves, eyes, heart, blood vessels, liver, bones, and joints. Symptoms of the late stage of syphilis include difficulty coordinating muscle movements, paralysis, numbness, gradual blindness, and dementia. This damage may be serious enough to cause death.
Neurosyphilis
Syphilis can invade the nervous system at any stage of infection, and causes a wide range of symptoms varying from no symptoms at all, to headache, altered behavior, and movement problems that look like Parkinson’s or Huntington’s disease. 7 This invasion of the nervous system is called “neurosyphilis.”
Note: Health departments report syphilis by its stage of infection, noting “neurological manifestations,” rather than using the term neurosyphilis. 3
HIV infection and syphilis symptoms
Individuals who are HIV-positive can develop symptoms very different from the symptoms described above, including hypopigmented skin rashes. 8 HIV can also increase the chances of developing syphilis with neurological involvement. 9
How does syphilis affect a pregnant woman and her baby?
The syphilis bacterium can infect the baby of a woman during her pregnancy. All pregnant women should be tested for syphilis at the first prenatal visit. The syphilis screening test should be repeated during the third trimester (28 to 32 weeks gestation) and at delivery in women who are at high risk for syphilis, live in areas of high syphilis morbidity, are previously untested, or had a positive screening test in the first trimester. 10
Depending on how long a pregnant woman has been infected, she may have a high risk of having a stillbirth (a baby born dead) or of giving birth to a baby who dies shortly after birth; untreated syphilis in pregnant women results in infant death in up to 40 percent of cases. 6 Any woman who delivers a stillborn infant after 20 week’s gestation should also be tested for syphilis.
An infected baby born alive may not have any signs or symptoms of disease. However, if not treated immediately, the baby may develop serious problems within a few weeks. Untreated babies may become developmentally delayed, have seizures, or die. All babies born to mothers who test positive for syphilis during pregnancy should be screened for syphilis and examined thoroughly for evidence of congenital syphilis. 10
For pregnant women only penicillin therapy can be used to treat syphilis and prevent passing the disease to her baby; treatment with penicillin is extremely effective (success rate of 98%) in preventing mother-to-child transmission. 11 Pregnant women who are allergic to penicillin should be referred to a specialist for desensitization to penicillin.
How is syphilis diagnosed?
The definitive method for diagnosing syphilis is visualizing the spirochete via darkfield microscopy. This technique is rarely performed today because it is a technologically difficult method. Diagnoses are thus more commonly made using blood tests. There are two types of blood tests available for syphilis: 1) nontreponemal tests and 2) treponemal tests.
Nontreponemal tests (e.g., VDRL and RPR) are simple, inexpensive, and are often used for screening. However, they are not specific for syphilis, can produce false-positive results, and, by themselves, are insufficient for diagnosis. VDRL and RPR should each have their antibody titer results reported quantitatively. Persons with a reactive nontreponemal test should receive a treponemal test to confirm a syphilis diagnosis. This sequence of testing (nontreponemal, then treponemal test) is considered the “classical” testing algorithm.
Treponemal tests (e.g., FTA-ABS, TP-PA, various EIAs, and chemiluminescence immunoassays) detect antibodies that are specific for syphilis. Treponemal antibodies appear earlier than nontreponemal antibodies and usually remain detectable for life, even after successful treatment. If a treponemal test is used for screening and the results are positive, a nontreponemal test with titer should be performed to confirm diagnosis and guide patient management decisions. Based on the results, further treponemal testing may be indicated. For further guidance, please refer to the 2010 STD Treatment Guidelines. 10 This sequence of testing (treponemal, then nontreponemal, test) is considered the “reverse” sequence testing algorithm.Reverse sequence testing can be more convenient for laboratories, but its clinical interpretation is problematic, as this testing sequence can identify individuals not previously described (e.g., treponemal test positive, nontreponemal test negative), making optimal management choices difficult. 12
Special note: Because untreated syphilis in a pregnant woman can infect and possibly kill her developing baby, every pregnant woman should have a blood test for syphilis. All women should be screened at their first prenatal visit. For patients who belong to communities and populations with high prevalence of syphilis and for patients at high risk, blood tests should also be performed during the third trimester (at 28–32 weeks) and at delivery. For further information on screening guidelines, please refer to the 2010 STD Treatment Guidelines. 10
All infants born to mothers who have reactive nontreponemal and treponemal test results should be evaluated for congenital syphilis. A quantitative nontreponemal test should be performed on infant serum and, if reactive, the infant should be examined thoroughly for evidence of congenital syphilis. Suspicious lesions, body fluids, or tissues (e.g., umbilical cord, placenta) should be examined by darkfield microscopy and/or special stains. Other recommended evaluations may include analysis of cerebrospinal fluid by VDRL, cell count and protein, CBC with differential and platelet count, and long-bone radiographs. For further guidance on evaluation of infants for congenital syphilis, please refer to the 2010 STD Treatment Guidelines. 10
What is the link between syphilis and HIV?
Genital sores caused by syphilis make it easier to transmit and acquire HIV infection sexually. There is an estimated 2- to 5-fold increased risk of acquiring HIV if exposed to that infection when syphilis is present. 13
Ulcerative STDs that cause sores, ulcers, or breaks in the skin or mucous membranes, such as syphilis, disrupt barriers that provide protection against infections. The genital ulcers caused by syphilis can bleed easily, and when they come into contact with oral and rectal mucosa during sex, increase the infectiousness of and susceptibility to HIV. Studies have observed that infection with syphilis was associated with subsequent HIV infection among MSM. 14, 15
Having other STDs can also indicate increased risk for becoming HIV infected. 14
What is the treatment for syphilis?
There are no home remedies or over-the-counter drugs that will cure syphilis, but syphilis is easy to cure in its early stages. A single intramuscular injection of long acting Benzathine penicillin G (2.4 million units administered intramuscularly) will cure a person who has primary, secondary or early latent syphilis. Three doses of long acting Benzathine penicillin G (2.4 million units administered intramuscularly) at weekly intervals is recommended for individuals with late latent syphilis or latent syphilis of unknown duration. Treatment will kill the syphilis bacterium and prevent further damage, but it will not repair damage already done.
Selection of the appropriate penicillin preparation is important to properly treat and cure syphilis. Combinations of some penicillin preparations (e.g., Bicillin C-R, a combination of benzathine penicillin and procaine penicillin) are not appropriate treatments for syphilis, as these combinations provide inadequate doses of penicillin. 16
Although data to support the use of alternatives to penicillin is limited, options for non-pregnant patients who are allergic to penicillin may include doxycycline, tetracycline, and for neurosyphilis, potentially probenecid. These therapies should be used only in conjunction with close clinical and laboratory follow-up to ensure appropriate serological response and cure. 10
Persons who receive syphilis treatment must abstain from sexual contact with new partners until the syphilis sores are completely healed. Persons with syphilis must notify their sex partners so that they also can be tested and receive treatment if necessary.
Who should be tested for syphilis?
Any person with signs or symptoms of primary infection, secondary infection, neurologic infection, or tertiary infection should be tested for syphilis.
Providers should routinely test persons who
- are pregnant;
- are members of an at-risk subpopulation (i.e., persons in correctional facilities and MSM);
- describe sexual behaviors that put them at risk for STDs (i.e., having unprotected vaginal, anal, or oral sexual contact; having multiple sexual partners; using drugs and alcohol, and engaging in commercial or coerced sex);
- have partner(s) who have tested positive for syphilis ;
- are sexually active and live in areas with high syphilis morbidity.
Will syphilis recur?
Syphilis does not recur. However, having syphilis once does not protect a person from becoming infected again. Even following successful treatment, people can be re-infected. Patients with signs or symptoms that persist or recur or who have a sustained fourfold increase in nontreponemal test titer probably failed treatment or were reinfected. These patients should be retreated.
Because chancres can be hidden in the vagina, rectum, or mouth, it may not be obvious that a sex partner has syphilis. Unless a person knows that their sex partners have been tested and treated, they may be at risk of being reinfected by an untreated partner. For further details on the management of sex partners, refer to the 2010 STD Treatment Guidelines. 10
How can syphilis be prevented?
Correct and consistent use of latex condoms can reduce the risk of syphilis only when the infected area or site of potential exposure is protected. However, a syphilis sore outside of the area covered by a latex condom can still allow transmission, so caution should be exercised even when using a condom. For persons who have latex allergies, synthetic non-latex condoms can be used but it is important to note that they have higher breakage rates than latex condoms. 17 Natural membrane condoms are not recommended for STD prevention. 18 Other individual-based interventions, such as the use of microbicide or male circumcision, do not prevent syphilis. 19
The surest way to avoid transmission of sexually transmitted diseases, including syphilis, is to abstain from sexual contact or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
Partner-based interventions include partner notification – a critical component in preventing the spread of syphilis. Sexual partners of infected patients should be considered at risk and provided treatment per the 2010 STD Treatment Guidelines. 10
Transmission of an STD, including syphilis, cannot be prevented by washing the genitals, urinating, and/or douching after sex. Any unusual discharge, sore, or rash, particularly in the groin area, should be a signal to refrain from having sex and to see a doctor immediately.
Avoiding alcohol and drug use may also help prevent transmission of syphilis because these activities may lead to risky sexual behavior. It is important that sex partners talk to each other about their HIV status and history of other STDs so that preventive action can be taken.
CDC
back-to-top
Trichomoniasis - CDC Fact Sheet
Most people who have trichomoniasis do not have any symptoms.
What is trichomoniasis?
Trichomoniasis (or “trich”) is a very common sexually transmitted disease (STD) that is caused by infection with a protozoan parasite called Trichomonas vaginalis. Although symptoms of the disease vary, most women and men who have the parasite cannot tell they are infected.
How common is trichomoniasis?
Trichomoniasis is considered the most common curable STD. In the United States, an estimated 3.7 million people have the infection, but only about 30% develop any symptoms of trichomoniasis. Infection is more common in women than in men, and older women are more likely than younger women to have been infected.
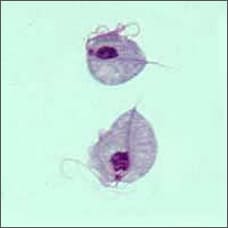
Two Trichomonas vaginalis parasites, magnified (seen under a microscope)How do people get trichomoniasis?
The parasite is passed from an infected person to an uninfected person during sex. In women, the most commonly infected part of the body is the lower genital tract (vulva, vagina, or urethra), and in men, the most commonly infected body part is the inside of the penis (urethra). During sex, the parasite is usually transmitted from a penis to a vagina, or from a vagina to a penis, but it can also be passed from a vagina to another vagina. It is not common for the parasite to infect other body parts, like the hands, mouth, or anus. It is unclear why some people with the infection get symptoms while others do not, but it probably depends on factors like the person’s age and overall health. Infected people without symptoms can still pass the infection on to others.
What are the signs and symptoms of trichomoniasis?
About 70% of infected people do not have any signs or symptoms. When trichomoniasis does cause symptoms, they can range from mild irritation to severe inflammation. Some people with symptoms get them within 5 to 28 days after being infected, but others do not develop symptoms until much later. Symptoms can come and go.
Men with trichomoniasis may feel itching or irritation inside the penis, burning after urination or ejaculation, or some discharge from the penis.
Women with trichomoniasis may notice itching, burning, redness or soreness of the genitals, discomfort with urination, or a thin discharge with an unusual smell that can be clear, white, yellowish, or greenish.
Having trichomoniasis can make it feel unpleasant to have sex. Without treatment, the infection can last for months or even years.
 STDs & Pregnancy
STDs & Pregnancy
What are the complications of trichomoniasis?
Trichomoniasis can increase the risk of getting or spreading other sexually transmitted infections. For example, trichomoniasis can cause genital inflammation that makes it easier to get infected with the HIV virus, or to pass the HIV virus on to a sex partner.
How does trichomoniasis affect a pregnant woman and her baby?
Pregnant women with trichomoniasis are more likely to have their babies too early (preterm delivery). Also, babies born to infected mothers are more likely to have an officially low birth weight (less than 5.5 pounds).
How is trichomoniasis diagnosed?
It is not possible to diagnose trichomoniasis based on symptoms alone. For both men and women, your primary care doctor or another trusted health care provider must do a check and a laboratory test to diagnose trichomoniasis.
What is the treatment for trichomoniasis?
Trichomoniasis can be cured with a single dose of prescription antibiotic medication (either metronidazole or tinidazole), pills which can be taken by mouth. It is okay for pregnant women to take this medication. Some people who drink alcohol within 24 hours after taking this kind of antibiotic can have uncomfortable side effects.
People who have been treated for trichomoniasis can get it again. About 1 in 5 people get infected again within 3 months after treatment. To avoid getting reinfected, make sure that all of your sex partners get treated too, and wait to have sex again until all of your symptoms go away (about a week). Get checked again if your symptoms come back.
How can trichomoniasis be prevented?
Using latex condoms correctly every time you have sex will help reduce the risk of getting or spreading trichomoniasis. However, condoms don’t cover everything, and it is possible to get or spread this infection even when using a condom.
The only sure way to prevent sexually transmitted infections is to avoid having sex entirely. Another approach is to talk about these kinds of infections before you have sex with a new partner, so that you can make informed choices about the level of risk you are comfortable taking with your sex life.
If you or someone you know has questions about trichomoniasis or any other STD, especially with symptoms like unusual discharge, burning during urination, or a sore in the genital area, check in with a health care provider and get some answers.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
www.cdc.gov/std
CDC-INFO Contact Center
1-800-CDC-INFO (1-800-232-4636)
TTY: (888) 232-6348
Contact CDC-INFO
Source: Centers for Disease Control and Prevention, HHS
back-to-top
Scabies Frequently Asked Questions (FAQs)
On this Page
- What is scabies?
- What is crusted (Norwegian) scabies?
- How soon after infestation do symptoms of scabies begin?
- What are the signs and symptoms of scabies infestation?
- How did I get scabies?
- How is scabies infestation diagnosed?
- How long can scabies mites live?
- Can scabies be treated?
- Who should be treated for scabies?
- How soon after treatment will I feel better?
- Did I get scabies from my pet?
- Can scabies be spread by swimming in a public pool?
- How can I remove scabies mites from my house or carpet?
- How can I remove scabies mites from my clothes?
- My spouse and I were diagnosed with scabies. After several treatments, he/she still has symptoms while I am cured. Why?
- If I come in contact with a person who has scabies, should I treat myself?
What is scabies?
Scabies is an infestation of the skin by the human itch mite (Sarcoptes scabiei var. hominis). The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. The most common symptoms of scabies are intense itching and a pimple-like skin rash. The scabies mite usually is spread by direct, prolonged, skin-to-skin contact with a person who has scabies.
Scabies is found worldwide and affects people of all races and social classes. Scabies can spread rapidly under crowded conditions where close body and skin contact is frequent. Institutions such as nursing homes, extended-care facilities, and prisons are often sites of scabies outbreaks. Child care facilities also are a common site of scabies infestations.
What is crusted (Norwegian) scabies?
Crusted scabies is a severe form of scabies that can occur in some persons who are immunocompromised (have a weak immune system), elderly, disabled, or debilitated. It is also called Norwegian scabies. Persons with crusted scabies have thick crusts of skin that contain large numbers of scabies mites and eggs. Persons with crusted scabies are very contagious to other persons and can spread the infestation easily both by direct skin-to-skin contact and by contamination of items such as their clothing, bedding, and furniture. Persons with crusted scabies may not show the usually signs and symptoms of scabies such as the characteristic rash or itching (pruritus). Persons with crusted scabies should receive quick and aggressive medical treatment for their infestation to prevent outbreaks of scabies.
How soon after infestation do symptoms of scabies begin?
If a person has never had scabies before, symptoms may take as long as 4-6 weeks to begin. It is important to remember that an infested person can spread scabies during this time, even if he/she does not have symptoms yet.
In a person who has had scabies before, symptoms usually appear much sooner (1-4 days) after exposure.
What are the signs and symptoms of scabies infestation?
The most common signs and symptoms of scabies are intense itching (pruritus), especially at night, and a pimple-like (papular) itchy rash. The itching and rash each may affect much of the body or be limited to common sites such as the wrist, elbow, armpit, webbing between the fingers, nipple, penis, waist, belt-line, and buttocks. The rash also can include tiny blisters (vesicles) and scales. Scratching the rash can cause skin sores; sometimes these sores become infected by bacteria.
Tiny burrows sometimes are seen on the skin; these are caused by the female scabies mite tunneling just beneath the surface of the skin. These burrows appear as tiny raised and crooked (serpiginous) grayish-white or skin-colored lines on the skin surface. Because mites are often few in number (only 10-15 mites per person), these burrows may be difficult to find. They are found most often in the webbing between the fingers, in the skin folds on the wrist, elbow, or knee, and on the penis, breast, or shoulder blades.
The head, face, neck, palms, and soles often are involved in infants and very young children, but usually not adults and older children.
Persons with crusted scabies may not show the usual signs and symptoms of scabies such as the characteristic rash or itching (pruritus).
How did I get scabies?
Scabies usually is spread by direct, prolonged, skin-to-skin contact with a person who has scabies. Contact generally must be prolonged; a quick handshake or hug usually will not spread scabies. Scabies is spread easily to sexual partners and household members. Scabies in adults frequently is sexually acquired. Scabies sometimes is spread indirectly by sharing articles such as clothing, towels, or bedding used by an infested person; however, such indirect spread can occur much more easily when the infested person has crusted scabies.
How is scabies infestation diagnosed?
Diagnosis of a scabies infestation usually is made based on the customary appearance and distribution of the rash and the presence of burrows. Whenever possible, the diagnosis of scabies should be confirmed by identifying the mite, mite eggs, or mite fecal matter (scybala). This can be done by carefully removing a mite from the end of its burrow using the tip of a needle or by obtaining skin scraping to examine under a microscope for mites, eggs, or mite fecal matter. It is important to remember that a person can still be infested even if mites, eggs, or fecal matter cannot be found; typically fewer than 10-15 mites can be present on the entire body of an infested person who is otherwise healthy. However, persons with crusted scabies can be infested with thousands of mites and should be considered highly contagious.
How long can scabies mites live?
On a person, scabies mites can live for as long as 1-2 months. Off a person, scabies mites usually do not survive more than 48-72 hours. Scabies mites will die if exposed to a temperature of 50°C (122°F) for 10 minutes.
Can scabies be treated?
Yes. Products used to treat scabies are called scabicides because they kill scabies mites; some also kill eggs. Scabicides to treat human scabies are available only with a doctor’s prescription; no "over-the-counter" (non-prescription) products have been tested and approved for humans.
Always follow carefully the instructions provided by the doctor and pharmacist, as well as those contained in the box or printed on the label. When treating adults and older children, scabicide cream or lotion is applied to all areas of the body from the neck down to the feet and toes; when treating infants and young children, the cream or lotion also is applied to the head and neck. The medication should be left on the body for the recommended time before it is washed off. Clean clothes should be worn after treatment.
In addition to the infested person, treatment also is recommended for household members and sexual contacts, particularly those who have had prolonged skin-to-skin contact with the infested person. All persons should be treated at the same time in order to prevent reinfestation. Retreatment may be necessary if itching continues more than 2-4 weeks after treatment or if new burrows or rash continue to appear.
Never use a scabicide intended for veterinary or agricultural use to treat humans!
Who should be treated for scabies?
Anyone who is diagnosed with scabies, as well as his or her sexual partners and other contacts who have had prolonged skin-to-skin contact with the infested person, should be treated. Treatment is recommended for members of the same household as the person with scabies, particularly those persons who have had prolonged skin-to-skin contact with the infested person. All persons should be treated at the same time to prevent reinfestation.
Retreatment may be necessary if itching continues more than 2-4 weeks after treatment or if new burrows or rash continue to appear.
How soon after treatment will I feel better?
If itching continues more than 2-4 weeks after initial treatment or if new burrows or rash continue to appear (if initial treatment includes more than one application or dose, then the 2-4 time period begins after the last application or dose), retreatment with scabicide may be necessary; seek the advice of a physician.
Did I get scabies from my pet?
No. Animals do not spread human scabies. Pets can become infested with a different kind of scabies mite that does not survive or reproduce on humans but causes "mange" in animals. If an animal with "mange" has close contact with a person, the animal mite can get under the person’s skin and cause temporary itching and skin irritation. However, the animal mite cannot reproduce on a person and will die on its own in a couple of days. Although the person does not need to be treated, the animal should be treated because its mites can continue to burrow into the person’s skin and cause symptoms until the animal has been treated successfully.
Can scabies be spread by swimming in a public pool?
Scabies is spread by prolonged skin-to-skin contact with a person who has scabies. Scabies sometimes also can be spread by contact with items such as clothing, bedding, or towels that have been used by a person with scabies, but such spread is very uncommon unless the infested person has crusted scabies.
Scabies is very unlikely to be spread by water in a swimming pool. Except for a person with crusted scabies, only about 10-15 scabies mites are present on an infested person; it is extremely unlikely that any would emerge from under wet skin.
Although uncommon, scabies can be spread by sharing a towel or item of clothing that has been used by a person with scabies.
How can I remove scabies mites from my house or carpet?
Scabies mites do not survive more than 2-3 days away from human skin. Items such as bedding, clothing, and towels used by a person with scabies can be decontaminated by machine-washing in hot water and drying using the hot cycle or by dry-cleaning. Items that cannot be washed or dry-cleaned can be decontaminated by removing from any body contact for at least 72 hours.
Because persons with crusted scabies are considered very infectious, careful vacuuming of furniture and carpets in rooms used by these persons is recommended.
Fumigation of living areas is unnecessary.
How can I remove scabies mites from my clothes?
Scabies mites do not survive more than 2-3 days away from human skin. Items such as bedding, clothing, and towels used by a person with scabies can be decontaminated by machine-washing in hot water and drying using the hot cycle or by dry-cleaning. Items that cannot be washed or dry-cleaned can be decontaminated by removing from any body contact for at least 72 hours.
My spouse and I were diagnosed with scabies. After several treatments, he/she still has symptoms while I am cured. Why?
The rash and itching of scabies can persist for several weeks to a month after treatment, even if the treatment was successful and all the mites and eggs have been killed. Your health care provider may prescribe additional medication to relieve itching if it is severe. Symptoms that persist for longer than 2 weeks after treatment can be due to a number of reasons, including:
- Incorrect diagnosis of scabies. Many drug reactions can mimic the symptoms of scabies and cause a skin rash and itching; the diagnosis of scabies should be confirmed by a skin scraping that includes observing the mite, eggs, or mite feces (scybala) under a microscope. If you are sleeping in the same bed with your spouse and have not become reinfested, and you have not retreated yourself for at least 30 days, then it is unlikely that your spouse has scabies.
- Reinfestation with scabies from a family member or other infested person if all patients and their contacts are not treated at the same time; infested persons and their contacts must be treated at the same time to prevent reinfestation.
- Treatment failure caused by resistance to medication, by faulty application of topical scabicides, or by failure to do a second application when necessary; no new burrows should appear 24-48 hours after effective treatment.
- Treatment failure of crusted scabies because of poor penetration of scabicide into thick scaly skin containing large numbers of scabies mites; repeated treatment with a combination of both topical and oral medication may be necessary to treat crusted scabies successfully.
- Reinfestation from items (fomites) such as clothing, bedding, or towels that were not appropriately washed or dry-cleaned (this is mainly of concern for items used by persons with crusted scabies); potentially contaminated items (fomites) should be machine washed in hot water and dried using the hot temperature cycle, dry-cleaned, or removed from skin contact for at least 72 hours.
- An allergic skin rash (dermatitis); or
- Exposure to household mites that cause symptoms to persist because of cross-reactivity between mite antigens.
If itching continues more than 2-4 weeks or if new burrows or rash continue to appear, seek the advice of a physician; retreatment with the same or a different scabicide may be necessary.
If I come in contact with a person who has scabies, should I treat myself?
No. If a person thinks he or she might have scabies, he/she should contact a doctor. The doctor can examine the person, confirm the diagnosis of scabies, and prescribe an appropriate treatment. Products used to treat scabies in humans are available only with a doctor’s prescription.
Sleeping with or having sex with any scabies infested person presents a high risk for transmission. The longer a person has skin-to-skin exposure, the greater is the likelihood for transmission to occur. Although briefly shaking hands with a person who has non-crusted scabies could be considered as presenting a relatively low risk, holding the hand of a person with scabies for 5-10 minutes could be considered to present a relatively high risk of transmission. However, transmission can occur even after brief skin-to-skin contact, such as a handshake, with a person who has crusted scabies. In general, a person who has skin-to-skin contact with a person who has crusted scabies would be considered a good candidate for treatment.
To determine when prophylactic treatment should be given to reduce the risk of transmission, early consultation should be sought with a health care provider who understands:
- the type of scabies (i.e. non-crusted vs crusted) to which a person has been exposed;
- the degree and duration of skin exposure that a person has had to the infested patient;
- whether the exposure occurred before or after the patient was treated for scabies; and,
- whether the exposed person works in an environment where he/she would be likely to expose other people during the asymptomatic incubation period. For example, a nurse or caretaker who works in a nursing home or hospital often would be treated prophylactically to reduce the risk of further scabies transmission in the facility.
Source: Centers for Disease Control and Prevention, HHS
back-to-top
Hepatitis A FAQs for Health Professionals
Index of Questions
± Overview and Statistics
± Hepatitis A Vaccination
± Hepatitis A and International Travel
± Postexposure Prophylaxis for Hepatitis A
Overview and Statistics
What is the case definition for acute Hepatitis A?
The clinical case definition for acute viral hepatitis is 1) discrete onset of symptoms (e.g., nausea, anorexia, fever, malaise, or abdominal pain) and 2) jaundice or elevated serum aminotransferase levels. Because the clinical characteristics are the same for all types of acute viral hepatitis, Hepatitis A diagnosis must be confirmed by a positive serologic test for immunoglobulin M (IgM) antibody to Hepatitis A virus, or the case must meet the clinical case definition and occur in a person who has an epidemiologic link with a person who has laboratory-confirmed Hepatitis A (i.e., household or sexual contact with an infected person during the 15–50 days before the onset of symptoms).
The case definition for acute Hepatitis A is available at the following link: Acute Hepatitis A
Additional guidance on viral hepatitis surveillance and case management is available at http://www.cdc.gov/hepatitis/SurveillanceGuidelines.htm.
How common is Hepatitis A virus (HAV) infection in the United States?
Hepatitis A rates in the United States have declined by 95% since Hepatitis A vaccine first became available in 1995.
In 2012, 1,562 acute symptomatic cases of Hepatitis A were reported; The overall incidence rate for 2012 was 0.5 cases per 100,000. After adjusting for asymptomatic infection and underreporting, the estimated number of new infections was 3,000.
How is HAV transmitted?
- Person-to-person transmission through the fecal-oral route (i.e., ingestion of something that has been contaminated with the feces of an infected person) is the primary means of HAV transmission in the United States. Most infections result from close personal contact with an infected household member or sex partner.
- Common-source outbreaks and sporadic cases also can occur from exposure to fecally contaminated food or water. Uncooked HAV-contaminated foods have been recognized as a source of outbreaks. Cooked foods also can transmit HAV if the temperature during food preparation is inadequate to kill the virus or if food is contaminated after cooking, as occurs in outbreaks associated with infected food handlers. Waterborne outbreaks are infrequent in developed countries with well-maintained sanitation and water supplies.
Who is at increased risk for acquiring HAV infection?
- Travelers to countries with high or intermediate endemicity of HAV infection
- Men who have sex with men
- Users of injection and non-injection illegal drugs
- Persons with clotting factor disorders
- Persons working with nonhuman primates
What are the signs and symptoms of HAV infection?
Some persons, particularly young children, are asymptomatic. When symptoms are present, they usually occur abruptly and can include the following:
- Fever
- Fatigue
- Loss of appetite
- Nausea
- Vomiting
- Abdominal pain
- Dark urine
- Clay-colored bowel movements
- Joint pain
- Jaundice
In children aged 70% of patients.
When symptoms occur, how long do they usually last?
Symptoms usually last less than 2 months, although 10%–15% of symptomatic persons have prolonged or relapsing disease for up to 6 months.
What is the incubation period for Hepatitis A?
The average incubation period for Hepatitis A is 28 days (range: 15–50 days).
How long does HAV survive outside the body? How can the virus be killed?
HAV can live outside the body for months, depending on the environmental conditions. The virus is killed by heating to >185 degrees F (>85 degrees C) for one minute. However, the virus can still be spread from cooked food if it is contaminated after cooking. Adequate chlorination of water, as recommended in the United States, kills HAV that enters the water supply.
Can Hepatitis A become chronic?
No. Hepatitis A does not become chronic.
Can persons become reinfected with HAV after recovering from Hepatitis A?
No. IgG antibodies to HAV, which appear early in the course of infection, provide lifelong protection against the disease.
How is HAV infection prevented?
Vaccination with the full, two-dose series of Hepatitis A vaccine is the best way to prevent HAV infection. Hepatitis A vaccine has been licensed in the United States for use in persons 12 months of age and older. The vaccine is recommended for persons who are more likely to get HAV infection or are more likely to get seriously ill if they get Hepatitis A, and for any person wishing to obtain immunity (see Who should be vaccinated against Hepatitis A?).
Immune globulin is available for short-term protection (approximately 3 months) against Hepatitis A, both pre- and post-exposure. Immune globulin must be administered within 2 weeks after exposure for maximum protection.
Good hygiene — including handwashing after using the bathroom, changing diapers, and before preparing or eating food — is also integral to Hepatitis A prevention, given that the virus is transmitted through the fecal–oral route. Environmental surfaces can be cleaned with a freshly prepared solution of 1:100 dilution of household bleach.
Hepatitis A Vaccination
Who should be vaccinated against Hepatitis A?
Hepatitis A vaccination is recommended for all children at age 1 year, for persons who are at increased risk for infection, for persons who are at increased risk for complications from Hepatitis A, and for any person wishing to obtain immunity. The following groups are recommended to receive Hepatitis A vaccination:
All children at age 1 year (i.e., 12–23 months). Children who have not been vaccinated by age 2 can be vaccinated at subsequent visits.
Children and adolescents ages 2–18 who live in states or communities where routine Hepatitis A vaccination has been implemented because of high disease incidence. Before 2006, when Hepatitis A vaccination was first recommended for all children at age 1 year, vaccination had been targeted to children living in states or communities that had historically high rates of Hepatitis A. States, counties, and communities with existing Hepatitis A vaccination programs for children aged 2–18 years are encouraged to maintain these programs. In those communities, new efforts focused on routine vaccination of children at age 1 year should enhance, not replace, ongoing programs directed at a broader population of children.
Persons traveling to or working in countries that have high or intermediate rates of Hepatitis A. Persons from developed countries who travel to developing countries are at high risk for Hepatitis A. The risk for Hepatitis A exists even for travelers to urban areas, those who stay in luxury hotels, and those who report that they have good hygiene and that they are careful about what they drink and eat (see Hepatitis A and International Travel for more information).
Men who have sex with men. Sexually active men (both adolescents and adults) who have sex with men should be vaccinated. Hepatitis A outbreaks among men who have sex with men have been reported frequently. Recent outbreaks have occurred in urban areas in the United States, Canada, and Australia.
Users of illegal injection and noninjection drugs. During the past two decades, outbreaks of Hepatitis A have been reported with increasing frequency among users of both injection and noninjection drugs (e.g., methamphetamine) in North America, Europe, and Australia.
Persons who have occupational risk for infection. Persons who work with HAV-infected primates or with HAV in a research laboratory setting should be vaccinated. No other groups have been shown to be at increased risk for HAV infection because of occupational exposure.
Persons who have chronic liver disease. Persons with chronic liver disease who have never had Hepatitis A should be vaccinated, as they have a higher rate of fulminant Hepatitis A (i.e., rapid onset of liver failure, often leading to death). Persons who are either awaiting or have received liver transplants also should be vaccinated.
Persons who have clotting-factor disorders. Persons who have never had Hepatitis A and who are administered clotting-factor concentrates, especially solvent detergent-treated preparations, should be vaccinated.
Household members and other close personal contacts of adopted children newly arriving from countries with high or intermediate hepatitis A endemicity.
Which Hepatitis A vaccines are licensed for use in the United States?
Two single-antigen Hepatitis A vaccines, HAVRIX® (manufactured by GlaxoSmithKline) and VAQTA® (manufactured by Merck & Co., Inc), are currently licensed in the United States. A combination vaccine, TWINRIX® (manufactured by GlaxoSmithKline), contains both HAV (in a lower dosage; see table) and Hepatitis B virus antigens. All are inactivated vaccines.
What are the dosages and schedules for Hepatitis A vaccines?
Licensed dosages and schedules for HAVRIX ® 1
| Age |
Dose (ELISA units)2 |
Volume (mL) |
No. of doses |
Schedule (mos)3 |
| 12 mos–18 yrs |
720 |
0.5 |
2 |
0,6-12 |
| ≥19 years |
1,440 |
1.0 |
2 |
0,6-12 |
1Hepatitis A vaccine, inactivated, GlaxoSmithKline.
2Enzyme-linked immunosorbent assay units.
30 months represents timing of the initial dose; subsequent numbers represent months after the initial dose.
|
Licensed dosages and schedules for VAQTA ® 1
|
| Age |
Dose (U.)2 |
Volume (mL) |
No. of doses |
Schedule (mos)3 |
| 12 mos–18 yrs |
25 |
0.5 |
2 |
0,6-18 |
| ≥19 years |
50 |
1.0 |
2 |
0,6-18 |
1Hepatitis A vaccine, inactivated, Merck & Co., Inc.
2Units.
30 months represents timing of the initial dose; subsequent numbers represent months after the initial dose.
TWINRIX ® 1 (HepAHepB) Vaccine Schedule (Not recommended for post exposure prophylaxis)
Licensed dosages and schedules for TWINRIX ® 1
| Age |
Dose (ELISA units)2 |
Volume (mL) |
No. of doses |
Schedule |
| ≥ 18 yrs |
720 |
1.0 |
3 |
0, 1, 6 mos |
| ≥ 18 yrs |
720 |
1.0 |
4 |
0, 7, 21–30 days + 12 mos3 |
1Combined Hepatitis A and Hepatitis B vaccine, inactivated, GlaxoSmithKline.
2Enzyme-linked immunosorbent assay units.
3This 4-dose schedule enables patients to receive 3 doses in 21 days; this schedule is used prior to planned exposure with short notice and requires a fourth dose at 12 months.
How long does protection from Hepatitis A vaccine last?
A recent review by an expert panel, which evaluated the projected duration of immunity from vaccination, concluded that protective levels of antibody to HAV could be present for at least 25 years in adults and at least 14–20 years in children.
Can Hepatitis A vaccine be administered concurrently with other vaccines?
Yes. Hepatitis B, diphtheria, poliovirus (oral and inactivated), tetanus, oral and intramuscular typhoid, cholera, Japanese encephalitis, rabies, and yellow fever vaccines and immune globulin can be given at the same time that Hepatitis A vaccine is given, but at a different injection site.
Can a patient receive the first dose of Hepatitis A vaccine from one manufacturer and the second (last) dose from another manufacturer?
Yes. Although studies have not been done to examine this issue, there is no reason to believe that using single-antigen vaccine from different manufacturers would be a problem.
What should be done if the second (last) dose of Hepatitis A vaccine is delayed?
The second dose should be administered as soon as possible. The first dose does not need to be readministered.
Can Hepatitis A vaccine be given during pregnancy?
The safety of Hepatitis A vaccination during pregnancy has not been determined; however, because the vaccine is produced from inactivated HAV, the theoretical risk to the developing fetus is expected to be low. The risk associated with vaccination, however, should be weighed against the risk for Hepatitis A in women who might be at high risk for exposure to HAV.
Can Hepatitis A vaccine be given to immunocompromised persons (e.g., persons on hemodialysis or persons with AIDS)?
Yes. Because Hepatitis A vaccine is inactivated, no special precautions need to be taken when vaccinating immunocompromised persons.
Is it harmful to administer an extra dose(s) of Hepatitis A or Hepatitis B vaccine or to repeat the entire vaccine series if documentation of vaccination history is unavailable?
No. If necessary, administering extra doses of Hepatitis A or Hepatitis B vaccine is not harmful.
Should prevaccination testing be performed before administering Hepatitis A vaccine?
Prevaccination testing is recommended only in specific circumstances to reduce the costs of vaccinating people who are already immune to Hepatitis A, including
- Persons who were born in geographic areas with high or intermediate prevalence of HAV infection
- Older adolescents and adults in certain population groups (i.e., American Indians, Alaska Natives, and Hispanics)
- Adults in groups that have a high prevalence of infection (e.g., injection drug users)
Prevaccination testing might also be warranted for all older adults. The decision to test should be based on 1) the expected prevalence of immunity, 2) the cost of vaccination compared with the cost of serologic testing, and 3) the likelihood that testing will not interfere with initiation of vaccination.
Should postvaccination testing be performed?
No. Postvaccination testing is not indicated because of the high rate of vaccine response among adults and children. In addition, not all testing methods approved for routine diagnostic use in the United States have the sensitivity to detect low, but protective, anti-HAV concentrations after vaccination.
Which groups do NOT need routine vaccination against Hepatitis A?
Food service workers. Foodborne Hepatitis A outbreaks are relatively uncommon in the United States; however, when they occur, intensive public health efforts are required for their control.
Although persons who work as food handlers have a critical role in common-source foodborne outbreaks, they are not at increased risk for Hepatitis A because of their occupation. Consideration may be given to vaccination of employees who work in areas where community-wide outbreaks are occurring and where state and local health authorities or private employers determine that such vaccination is cost-effective.
Sewage workers. In the United States, no work-related outbreaks of Hepatitis A have been reported among workers exposed to sewage.
Health care workers. Health care workers are not at increased risk for Hepatitis A. If a patient with Hepatitis A is admitted to the hospital, routine infection-control precautions will prevent transmission to hospital staff.
Children under 12 months of age. Because of the limited experience with Hepatitis A vaccination among children in this age group, the vaccine is not currently licensed for children age
Child care center attendees. The frequency of outbreaks of Hepatitis A is not high enough in this setting to warrant routine Hepatitis A vaccination of staff. Hepatitis A vaccination is recommended for all children at 1 year of age, including children attending child day care centers.
Residents of institutions for developmentally disabled persons. Historically, Hepatitis A virus infections were common among persons with developmental disabilities living in institutions. The occurrence of HAV infection has diminished, and routine vaccination against Hepatitis A is no longer recommended for this population.
Hepatitis A and International Travel
Who should receive protection against Hepatitis A before travel?
All susceptible persons traveling to or working in countries that have high or intermediate rates of Hepatitis A should be vaccinated or receive immune globulin (IG) before traveling. Persons from developed countries who travel to developing countries are at high risk for Hepatitis A. The risk for Hepatitis A exists even for travelers to urban areas, those who stay in luxury hotels, and those who report that they have good hygiene and that they are careful about what they drink and eat. For more information on international travel and HAV, see CDC's travel page at http://wwwn.cdc.gov/travel/yellowBookCh4-HepA.aspx .
How soon before travel should the first dose of Hepatitis A vaccine be given?
The first dose of Hepatitis A vaccine should be administered as soon as travel is considered.
Previously, Hepatitis A vaccination was recommended to be administered at least 2–4 weeks before departure to an area with intermediate or high rates of Hepatitis A. Travelers who were departing in less than 2 weeks were recommended to receive receive immune globulin (IG) for short-term protection.
However, on the basis of data indicating that immune globulin and vaccine have equivalent postexposure efficacy among healthy persons aged ≤40 years, the Advisory Committee on Immunization Practices (ACIP) has amended its guidelines for Hepatitis A vaccination for travelers. ACIP now recommends that one dose of single-antigen Hepatitis A vaccine administered at any time before departure may provide adequate protection for most healthy persons.
For optimal protection, older adults, immunocompromised persons, and persons with chronic liver disease or other chronic medical conditions who are planning to depart in ≤2 weeks should receive the initial dose of vaccine and also can simultaneously be administered IG (0.02 mL/kg) at a separate anatomic injection site.
What should be done if a traveler cannot receive Hepatitis A vaccine?
Travelers who are allergic to a vaccine component, who elect not to receive vaccine, or who are aged
What should be done for travelers less than 12 months of age?
Immune globulin is recommended because Hepatitis A vaccine is currently not approved for use in this age group.
Postexposure Prophylaxis for Hepatitis A
What are the current CDC guidelines for postexposure protection against Hepatitis A?
Until recently, an injection of immune globulin (IG) was the only recommended way to protect people after they have been exposed to Hepatitis A virus. In June 2007, U.S. guidelines were revised to allow for Hepatitis A vaccine to be used after exposure to prevent infection in healthy persons aged 1–40 years.
Persons who have recently been exposed to HAV and who have not been vaccinated previously should be administered a single dose of single-antigen Hepatitis A vaccine or IG (0.02 mL/kg) as soon as possible, within 2 weeks after exposure. The guidelines vary by age and health status:
- For healthy persons aged 12 months–40 years, single-antigen Hepatitis A vaccine at the age-appropriate dose is preferred to IG because of the vaccine’s advantages, including long-term protection and ease of administration, as well as the equivalent efficacy of vaccine to IG.
- For persons aged 40 years and older, IG is preferred because of the absence of information regarding vaccine performance in this age group and because of the more severe manifestations of Hepatitis A in older adults. The magnitude of the risk of HAV transmission from the exposure should be considered in decisions to use vaccine or IG in this age group.
- Vaccine can be used if IG cannot be obtained.
- IG should be used for children aged less than12 months, immunocompromised persons, persons with chronic liver disease, and persons who are allergic to the vaccine or a vaccine component (see Footnote).
Footnote:
- CDC does not have official guidance to define all subgroups of persons recommended to receive IG.
- IG is indicated for persons at increased risk of severe or fatal hepatitis A infection. These persons include adults older than 40 years of age, particularly adults 75 years and older, persons with chronic liver disease (e.g., cirrhosis), and those who are immunocompromised.
- IG is indicated for persons with decreased response to hepatitis A vaccine. Based on available data such persons include those with HIV/AIDs, persons undergoing hemodialysis, recipients of solid organ, bone marrow or stem cell transplants, persons with chronic liver disease (e.g., cirrhosis), and other patients unlikely to develop an adequate immune response. Also, antibody response after a single dose of hepatitis A vaccine in persons older than 40 years may be reduced, but data are limited.
- Immunocompromised persons generally are incapable of developing a normal immune response, usually as a result of disease, malnutrition, or immunosuppressive therapy. IG is indicated for patients who might include those receiving high dose steroids, chemotherapy, immunomodulators, and those who have primary immunodeficiency conditions. Clinical guidance should be obtained if the immune status is unclear.
Who requires protection (i.e., IG or Hepatitis A vaccine) after exposure to HAV?
Close personal contacts. Close personal contacts of persons with serologically confirmed Hepatitis A (i.e., through a blood test), including:
- Household and sex contacts
- Persons who have shared illicit drugs with someone with Hepatitis A
Consideration should also be given to providing IG or Hepatitis A vaccine to persons with other types of ongoing, close personal contact with a person with Hepatitis A (e.g., a regular babysitter or caretaker).
Child-care center staff, attendees, and attendees' household members
- Postexposure prophylaxis (PEP) should be administered to all previously unvaccinated staff and attendees of child care centers or homes if 1) one or more cases of Hepatitis A are recognized in children or employees or 2) cases are recognized in two or more households of center attendees.
- In centers that provide care only to older children who no longer wear diapers, PEP need be administered only to classroom contacts of the index patient (i.e., not to children or staff in other classrooms).
- When an outbreak occurs (i.e., Hepatitis A cases in three or more families), PEP should also be considered for members of households that have diaper-wearing children attending the center.
Persons exposed to a common source, such as an infected food handler. If a food handler receives a diagnosis of Hepatitis A, post-exposure prophylaxis (PEP) should be administered to other food handlers at the same establishment. Because transmission to patrons is unlikely, PEP administration to patrons typically is not indicated but may be considered if 1) during the time when the food handler was likely to be infectious, the food handler both directly handled uncooked foods or foods after cooking and had diarrhea or poor hygienic practices, and 2) patrons can be identified and treated within 2 weeks of exposure.
In settings in which repeated exposures to HAV might have occurred, such as institutional cafeterias, stronger consideration of PEP use might be warranted.
If a case of Hepatitis A is found in a school, hospital, or office setting, what should be done?
If a single case of Hepatitis A is identified in a school (other than a child care setting in which children wear diapers), office, or other work setting, and if the source of infection is outside the school or work setting, PEP (i.e., injection of IG or Hepatitis A vaccine) is not routinely recommended. Similarly, when a person who has Hepatitis A is admitted to a hospital, staff should not routinely be administered PEP; instead, careful hygienic practices should be emphasized.
However, if it is determined that Hepatitis A has been spread among students in a school or among patients and staff in a hospital, PEP should be administered to unvaccinated persons who have had close contact with an infected person.
Source: Centers for Disease Control and Prevention, HHS
back-to-top
STDs & Pregnancy - CDC Fact Sheet
Women who are pregnant can become infected with the same sexually transmitted diseases (STDs) as women who are not pregnant. Pregnant women should ask their doctors about getting tested for STDs, since some doctors do not routinely perform these tests.

Can pregnant women become infected with STDs?
Women who are pregnant can become infected with the same sexually-transmitted diseases (STDs) as women who are not pregnant. Pregnancy does not provide women or their babies any additional protection against STDs. Many STDs are ‘silent,’ or have no symptoms, so women may not know they are infected. A pregnant woman should be tested for STDs, including HIV (the virus that causes AIDS), as a part of her medical care during pregnancy. The results of an STD can be more serious, even life-threatening, for a woman and her baby if the woman becomes infected while pregnant. It is important that women be aware of the harmful effects of STDs and how to protect themselves and their children against infection. Sexual partners of infected women should also be tested and treated.
How do STDs affect a pregnant woman and her baby?
STDs can complicate pregnancy and may have serious effects on both a woman and her developing baby. Some of these problems may be seen at birth; others may not be discovered until months or years later. In addition, it is well known that infection with an STD can make it easier for a person to get infected with HIV1. Most of these problems can be prevented if the mother receives regular medical care during pregnancy. This includes tests for STDs starting early in pregnancy and repeated close to delivery, as needed.
Human Immunodeficiency Virus
Human immunodeficiency virus (HIV) is the virus that causes acquired immune deficiency syndrome, or AIDS. HIV destroys specific blood cells that are crucial to helping the body fight diseases. According to CDC’s 2011 HIV surveillance data, women make up 25% of all adults and adolescents living with diagnosed HIV infection in the United States2. The most common ways that HIV passes from mother to child are during pregnancy, labor and delivery, or through breastfeeding. However, when HIV is diagnosed before or during pregnancy and appropriate steps are taken, the risk of mother-to-child transmission can be lowered to less than 2%3. HIV testing is recommended for all pregnant women. A mother who knows early in her pregnancy that she is HIV-positive has more time to consult with her healthcare provider and decide on effective ways to protect her health and that of her unborn baby.
Syphilis
Syphilis is primarily a sexually transmitted disease, but may be passed to a baby by an infected mother during pregnancy. Passing syphilis to a developing baby can lead to serious health problems. Syphilis has been linked to premature births, stillbirths and, in some cases, death shortly after birth7. Untreated infants that survive tend to develop problems in multiple organs, including the brain, eyes, ears, heart, skin, teeth, and bones. Screening for syphilis should be performed in all pregnant women during their first prenatal medical visit and repeated in the third trimester, if the patient is considered to be at high risk.
Hepatitis B
Hepatitis B  is a liver infection caused by the hepatitis B virus (HBV). A mother can pass the infection to her baby during pregnancy. While the risk of an infected mother passing HBV to her baby varies depending on when she becomes infected, the greatest risk happens when mothers become infected close to the time of delivery14 Infected newborns also have a high risk (up to 90%) of becoming chronic (lifelong) HBV carriers themselves15. Infants who have a lifelong infection with HBV are at an increased risk for developing chronic liver disease or liver cancer later in life. Approximately one in four infants who develop chronic HBV infection will eventually die from chronic liver disease13. Mother-to-child transmission of HBV can be prevented by screening pregnant women for the infection and providing treatment to at-risk infants shortly after birth. Information on mother-to-child transmission of HBV can be found at http://www.cdc.gov/hepatitis/HBV/PerinatalXmtn.htm.
is a liver infection caused by the hepatitis B virus (HBV). A mother can pass the infection to her baby during pregnancy. While the risk of an infected mother passing HBV to her baby varies depending on when she becomes infected, the greatest risk happens when mothers become infected close to the time of delivery14 Infected newborns also have a high risk (up to 90%) of becoming chronic (lifelong) HBV carriers themselves15. Infants who have a lifelong infection with HBV are at an increased risk for developing chronic liver disease or liver cancer later in life. Approximately one in four infants who develop chronic HBV infection will eventually die from chronic liver disease13. Mother-to-child transmission of HBV can be prevented by screening pregnant women for the infection and providing treatment to at-risk infants shortly after birth. Information on mother-to-child transmission of HBV can be found at http://www.cdc.gov/hepatitis/HBV/PerinatalXmtn.htm.
Hepatitis C
Hepatitis C is a liver infection caused by the hepatitis C virus (HCV), and can be passed from an infected mother to her child during pregnancy. Overall, an infected mother will pass the infection to her baby 10% of the time, but the chances are higher in certain subgroups, such as women who are also infected with HIV16. Regular testing of pregnant women for HCV is not recommended, however, it should be considered for individuals who have risk factors known to be linked to HCV, including injection drug use. In some studies, infants born to HCV-infected women have been shown to have an increased risk for being small for gestational age, premature, and having a low birth weight15. Newborn infants with HCV infection usually do not have symptoms, and a majority will clear the infection without any medical help. Liver disease tends to move forward more slowly in children infected with hepatitis C and they respond slightly better to treatment compared to adults.
Chlamydia
Chlamydia is the most common sexually transmitted bacterium in the United States4. Although the majority of chlamydial infections do not have symptoms, pregnant women may have abnormal vaginal discharge, bleeding after sex, or itching/burning with urination. Untreated chlamydial infection has been linked to problems during pregnancy, including preterm labor, premature rupture of the membranes surrounding the baby in the uterus, and low birth weight5. The newborn may also become infected during delivery as the baby passes through the birth canal. Neonatal (newborn) infections lead primarily to eye and lung infections. All pregnant women should be tested for chlamydia at their first prenatal visit. Repeat testing in the third trimester should be done for women at high risk.
Gonorrhea
Gonorrhea is a common STD in the United States. Untreated gonococcal infection in pregnancy has been linked to miscarriages, premature birth and low birth weight, premature rupture of the membranes surrounding the baby in the uterus, and infection of the fluid that surrounds the baby during pregnancy6. Gonorrhea can also infect an infant during delivery as the infant passes through the birth canal. If untreated, infants can develop eye infections. Because gonorrhea can cause problems in both the mother and her baby, it is important to accurately identify the infection, treat with effective antibiotics, and closely follow up to make sure that the infection has been cured.
Bacterial Vaginosis
Bacterial vaginosis (BV), a common cause of vaginal discharge in women of childbearing age, is a condition in which the ‘good’ and ‘bad’ bacteria in the vagina are out of balance. BV is often not considered an STD, but it is linked to sexual activity. There may be no symptoms or a woman may complain of a foul-smelling, fishy, vaginal discharge. BV during pregnancy has been linked to serious pregnancy complications, including premature rupture of the membranes surrounding the baby in the uterus, preterm labor, premature birth, infection of the fluid that surrounds the baby, as well as infection of the mother’s uterus after delivery8. Testing all pregnant women for bacterial vaginosis is not currently recommended. However, there is some evidence to support testing and treating BV among women at high risk for preterm delivery9-11. There are no known direct effects of BV on the newborn.
Trichomoniasis
Vaginal infection due to the parasite Trichomonas vaginalis is a very common STD. Symptoms can vary widely among those women infected. Although some women report no symptoms, others complain of itching, foul odor, discharge, and bleeding after sex. Pregnant women are not usually screened for the infection. However, pregnant women with abnormal vaginal discharge should be evaluated for Trichomonas vaginalis and treated appropriately. Infection in pregnancy has been linked to premature rupture of the membranes surrounding the baby in the uterus, preterm birth, and low birth weight infants12. Rarely, the female newborn can get the infection when passing through the birth canal during delivery and have vaginal discharge after birth.
Herpes Simplex Virus
Herpes Simplex Virus (HSV) is a virus that has two distinct types, HSV-1 and HSV-2. Infections of the newborn can be of either type, but most are caused by HSV-2. Overall the symptoms of genital herpes are similar in pregnant and non-pregnant women; however, the major concern regarding HSV infection relates to complications linked to infection of the newborn. Although transmission may occur during pregnancy and after delivery, 80 - 90% of HSV infections in newborns occur when the baby passes through the mother’s infected birth canal18. HSV infection can have very serious effects on newborns, especially if the mother’s first outbreak occurred late in pregnancy (third trimester). Women who are infected for the first time in late pregnancy have a high risk of infecting their baby. Cesarean section is recommended for all women in labor with active genital herpes lesions or early symptoms, such as vulvar pain and itching19-20.
Human Papillomavirus
Human papillomaviruses (HPV) are viruses that most commonly involve the lower genital tract, including the cervix (opening to the womb), vagina, and external genitalia. Genital warts are symptoms of HPV infection that can be seen, and they frequently increase in number and size during pregnancy. Genital warts often appear as small cauliflower-like clusters which may burn or itch. If a woman has genital warts during pregnancy, treatment may be delayed until after delivery. When large or spread out, genital warts can complicate a vaginal delivery. In cases where there are large genital warts that are blocking the birth canal, a cesarean section may be recommended. Infection of the mother may be linked to the development of laryngeal papillomatosis in the newborn. This is a rare growth in the larynx (voice box) that is not cancer17.
Should pregnant women be tested for STDs?
Screening and treating pregnant women for STDs is a vital way to prevent serious health complications to both mother and baby that may otherwise happen with infection. The sooner a woman begins receiving medical care during pregnancy, the better the health outcomes will be for herself and her unborn baby. The Centers for Disease Control and Prevention’s 2010 STD Treatment Guidelines recommend screening pregnant women for STDs1. The CDC screening recommendations are incorporated into the recommendations below.
| Disease |
CDC Recommendation |
| Chlamydia |
Screen all pregnant women at first prenatal visit; 3rd trimester rescreen if younger than 25 years of age and/or high risk group |
| Gonorrhea |
Screen all pregnant women at risk at first prenatal visit; 3rd trimester rescreen women at continued high risk
Risk factors include: women younger than 25 years, living in a high morbidity area, previous GC infection, other STDs, new or multiple sex partners, inconsistent condom use, commercial sex work, drug use
|
| Syphilis |
Screen all pregnant women at first prenatal visit; during 3rd trimester rescreen women who are at high risk for syphilis or who live in areas with high numbers of syphilis cases, and/or those who were not previously tested or had a positive test in the first trimester |
| Bacterial Vaginosis |
Test pregnant women who have symptoms or are at high risk for preterm labor |
| Trichomoniasis |
Test pregnant women with symptoms |
| Herpes (HSV) |
Test pregnant women with symptoms |
| HIV |
Screen all pregnant women at first prenatal visit; rescreening in the third trimester recommended for women at high risk for getting HIV infection |
| Hepatitis B |
Screen all pregnant women at first prenatal visit
Retest those who were not screened prenatally, those who engage in behaviors that put them at high risk for infection and those with signs or symptoms of hepatitis at the time of admission to the hospital for delivery
Risk factors include: having had more than one sex partner in the previous six months, evaluation or treatment for an STD, recent or current injection-drug use, and an HBsAg-positive sex partner
|
| Human Papillomavirus |
There is not enough evidence to make a recommendation |
| Hepatitis C |
All pregnant women at high risk should be tested at first prenatal visit |

Pregnant women should ask their doctors about getting tested for these STDs. It is also important that pregnant women discuss any symptoms they are experiencing and any high-risk sexual behavior that they engage in, since some doctors do not routinely perform these tests. Even if a woman has been tested in the past, she should be tested again when she becomes pregnant.
Can STDs be treated during pregnancy?
STDs, such as chlamydia, gonorrhea, syphilis, trichomoniasis and BV can all be treated and cured with antibiotics that are safe to take during pregnancy. STDs that are caused by viruses, like genital herpes, hepatitis B, hepatitis C, or HIV cannot be cured. However, in some cases these infections can be treated with antiviral medications or other preventive measures to reduce the risk of passing the infection to the baby. If a woman is pregnant or considering pregnancy she should be tested so she can take steps to protect herself and her baby.
How can pregnant women protect themselves against infection?
Latex male condoms, when used consistently and correctly, can reduce the risk of getting or giving STDs and HIV. The surest way to avoid STDs and HIV is to abstain from vaginal, anal, and oral sex or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
Glossary of Terms
- Preterm labor – Labor that starts after 20 weeks but before the end of the 37th week of pregnancy.
- Premature birth – Birth of a baby before the 37th week of pregnancy.
- Premature rupture of membranes – Rupture of the membranes surrounding the baby in the uterus before the start of labor.
- Low birth weight – Birth weight of less than 5.5 pounds.
- Miscarriage – Death of the fetus before the 20th week of pregnancy.
- Stillbirth – Death of the fetus after the 20th week of pregnancy.
- Gestational age – Gestation is the period of time between conception and birth during which the fetus grows and develops inside the mother’s womb. Gestational age is the time measured from the first day of the mother’s last menstrual cycle to the current date, and it is measured in weeks.
Where can I get more information?
Division of STD Prevention (DSTDP)
Centers for Disease Control and Prevention
www.cdc.gov/std
Resources:
CDC National Prevention Information Network (NPIN)
P.O. Box 6003
Rockville, MD 20849-6003
E-mail: npin-info@cdc.gov
Source: Centers for Disease Control and Prevention, HHS
back-to-top














 Over-the-counter medicine. Medicine with this icon can be bought without a prescription.
Over-the-counter medicine. Medicine with this icon can be bought without a prescription.